LSECtin在小鼠肝硬化模型中减弱肝脏Th17的扩张,并通过LAG-3受体发出信号
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景,肝硬化进展过程中AimsLSECtin下调与适应性t细胞扩增有关。我们分析了LSECtin在实验性肝硬化模型中调节Th17增殖的分子机制。方法采用ccl4诱导肝硬化的转基因小鼠Clec4g/LSECtin过表达模型(Clec4g KI)。评估细胞死亡、肝功能和炎症标志物(n = 6/组)。我们还分析了th17分化脾脏细胞(n = 5)中LSECtin-LAG3信号的表达,以及肝硬化患者肝脏中LAG3的表达。结果Clec4g-KI小鼠在肝硬化期间密度测量的LSECtin表达显著下调(0.6±0.2 AU比2.3±0.6 AU, p = 0.001)。肝硬化小鼠过表达LSECtin可减轻组织损伤,改善肝酶水平(丙氨酸转氨酶:152.7±66.3 vs. 76.33±13.33 U/L, p = 0.004)。细胞死亡通路分析显示,凋亡标志物Casp3和Casp8无差异,但坏死中间体Mlkl和Ripk3水平降低。LSECtin过表达降低了肝硬化动物肝脏白细胞浸润,增强了炎症性Rorgt+/IL-17+(2.4±0.8 vs. 7.7±3.5% CD4+, p = 0.043)向调节性Foxp3+/IL-10+细胞(3.6±0.9 vs. 1.2±0.7% CD4+, p = 0.047)的分化。LSECtin与LAG-3在极化脾源性CD4+ T细胞上相互作用,通过抑制Stat3和Zap70通路抑制Th17分化(10.5±2.7 vs. 6.6±2.1% CD4+, p = 0.037)。LSECtin在LAG3阻断期间没有抑制Th17亚群扩增(6.6±2.1 vs. 14.2±4.0% CD4+, p = 0.005)。与对照组相比,肝硬化患者肝脏中表达lag -3的Th17细胞增加(3.2±1.4比0.1±0.1细胞/mm2, p = 0.001)。结论我们建立了一种有价值的LSECtin过表达小鼠模型,以评估其对肝硬化肝脏炎症的影响。LAG-3是LSECtin抑制Th17分化的分子机制。鉴于LSECtin调节Th谱和减少促炎细胞死亡的能力,肝脏定向分子方法恢复其表达可能是肝硬化治疗的兴趣。影响和意义slsectin下调与肝硬化期间Th17细胞亚群的扩大有关。研究结果强调了LSECtin在调节免疫反应和减轻肝硬化肝损伤中的关键作用。通过靶向肝脏定向分子干预恢复LSECtin表达可能与肝硬化的治疗相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
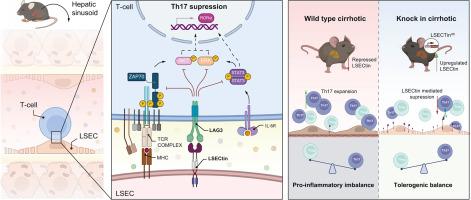
LSECtin attenuates hepatic Th17 expansion in a murine model of cirrhosis and signals through the LAG-3 receptor
Background & Aims
LSECtin downregulation during cirrhosis progression is associated with adaptive T-cell expansion. We analyzed the molecular mechanism for LSECtin modulation of Th17 proliferation in an experimental cirrhosis model.
Methods
A transgenic mouse model of Clec4g/LSECtin overexpression (Clec4g KI) was subjected to CCl4-induced cirrhosis. Cell death, liver function, and inflammation markers (n = 6/group) were evaluated. LSECtin-LAG3 signaling in Th17-differentiated spleen cells (n = 5) and LAG3 expression in livers of human individuals with cirrhosis (n = 6) were also characterized.
Results
Densitometred LSECtin expression was significantly downregulated during cirrhosis in wild-type but not Clec4g-KI mice (0.6 ± 0.2 vs. 2.3 ± 0.6 AU, p = 0.001). LSECtin overexpression in cirrhotic mice resulted in less histological damage and improved liver enzyme levels (alanine aminotransferase: 152.7 ± 66.3 vs. 76.33 ± 13.33 U/L, p = 0.004). Cell-death pathway analysis revealed no differences in apoptosis markers Casp3 and Casp8 but reduced levels of necroptotic intermediates Mlkl and Ripk3. LSECtin overexpression reduced hepatic leukocyte infiltration and enriched the differentiation from inflammatory Rorgt+/IL-17+ (2.4 ± 0.8 vs. 7.7 ± 3.5% CD4+, p = 0.043) to regulatory Foxp3+/IL-10+ cells (3.6 ± 0.9 vs. 1.2 ± 0.7% CD4+, p = 0.047) in cirrhotic animals. LSECtin interacted with LAG-3 on polarized spleen-derived CD4+ T cells, inhibiting Th17 differentiation (10.5 ± 2.7 vs. 6.6 ± 2.1% CD4+, p = 0.037) by suppressing Stat3 and Zap70 pathways. LSECtin did not restrain the Th17 subset expansion during LAG3 blockade (6.6 ± 2.1 vs. 14.2 ± 4.0% CD4+, p = 0.005). Livers of human individuals with cirrhosis showed increased LAG-3-expressing Th17 cells compared with controls (3.2 ± 1.4 vs. 0.1 ± 0.1 cells/mm2, p = 0.001).
Conclusions
We have established a valuable murine model of LSECtin overexpression to assess its impact on hepatic inflammation during cirrhosis. LAG-3 was identified as the molecular mechanism by which LSECtin attenuates Th17 differentiation. Given LSECtin capacity to modulate the Th profile and decrease proinflammatory cell death, liver-directed molecular approaches to restore its expression may be of therapeutic interest during cirrhosis.
Impact and implications
LSECtin downregulation is associated with the expansion of Th17 cell subpopulation during cirrhosis. Findings highlight the crucial role of LSECtin in regulating immune responses and mitigating liver damage in cirrhosis. Restoring LSECtin expression through targeted liver-directed molecular interventions may be of therapeutic relevance in cirrhosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


