内皮c-Maf通过调节染色质可及性抑制致病性微血管细胞亚群来预防masld样肝纤维化
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景,肝窦内皮细胞(LSECs)是肝脏血管生态位中高度特化的组成部分,通过血管分泌信号调节肝功能和疾病发病机制。最近,我们发现GATA4是控制LSEC发展和防止肝纤维化的关键转录因子。由于转录因子c-Maf在GATA4缺失的LSEC中被强烈下调,我们假设c-Maf可能是GATA4在LSEC分化和肝纤维化发生中的重要下游效应物。方法制备lsc特异性Maf缺乏的scec4g - icre /Maffl/fl (MafLSEC-KO)小鼠,对其肝组织进行组织学分析。分离LSECs进行bulk RNA-seq、ATAC-seq和单细胞(sc) RNA-seq分析。饲喂MASH日粮后对肝脏进行MafLSEC-KO分析。在已发表的人类scRNA-seq数据中分析了MAF及其靶点的表达。结果内皮细胞Maf缺乏导致肝周纤维化(天狼星红0.46% vs. 2.92%;p <0.05),但不影响代谢肝分区,并伴有从窦状向连续内皮细胞身份的转变,这种转变在饲喂MASH日粮后加剧(p <0.01)。内皮细胞Maf缺乏导致LSEC增殖(p <0.05),促纤维化血管分泌因子Pdgfb、Igfbp5、Flrt2和Cxcl12的表达,其中Flrt2 (p <0.01)和Cxcl12 (p <0.001)在体外激活肝星状细胞。scRNA-seq显示,分带的LSEC亚群被毛细血管化的、增殖的、发芽的和分泌的内皮细胞亚群所取代,这些细胞亚群促进了肝纤维化和血管生成。这种LSEC基因表达和分化的根本失调是由Maf缺失后染色质可及性和转录因子活性的变化引起的。值得注意的是,人肝硬化中内皮细胞MAF的表达也显著降低(p <0.0001)。结论肝内皮c-Maf对代谢功能障碍相关的脂肪性肝炎样肝纤维化具有保护作用,并通过控制染色质开口调节内皮细胞的分化和分带。影响和意义这项工作建立在肝窦内皮细胞在肝功能和疾病中的已知重要性的基础上。在这里,转录因子c-Maf被认为是维持肝窦内皮细胞正常分化和分带的主要调节因子,从而防止肝纤维化/肝硬化的发生。这些发现对于关注肝病的研究人员和临床医生来说意义重大,因为它们为治疗干预提供了潜在的新目标。这些发现可以指导新的预防治疗方案和抗纤维化治疗方案以及肝脏修复策略的发展,使患者,临床医生和肝脏疾病管理的决策者受益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
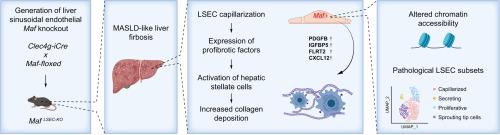
Endothelial c-Maf prevents MASLD-like liver fibrosis by regulating chromatin accessibility to suppress pathogenic microvascular cell subsets
Background & Aims
Liver sinusoidal endothelial cells (LSECs) are highly specialized components of the hepatic vascular niche, regulating liver function and disease pathogenesis through angiocrine signaling. Recently, we identified GATA4 as a key transcription factor controlling LSEC development and protecting against liver fibrosis. As the transcription factor c-Maf was strongly downregulated in Gata4-deficient LSECs, we hypothesized that c-Maf might be an important downstream effector of GATA4 in LSEC differentiation and liver fibrogenesis.
Methods
Clec4g-iCre/Maffl/fl (MafLSEC-KO) mice with LSEC-specific Maf deficiency were generated and liver tissue was analyzed histologically. LSECs were isolated for bulk RNA-seq, ATAC-seq, and single-cell (sc) RNA-seq analysis. MafLSEC-KO livers were analyzed after MASH diet feeding. The expression of MAF and its targets was analyzed in published human scRNA-seq data.
Results
Endothelial Maf deficiency resulted in perisinusoidal liver fibrosis (Sirius red 0.46% vs. 2.92%; p <0.05) without affecting metabolic liver zonation, accompanied by a switch from sinusoidal to continuous endothelial cell identity, which was aggravated upon MASH diet feeding (p <0.01). Furthermore, endothelial Maf deficiency caused LSEC proliferation (p <0.05) and expression of profibrotic angiocrine factors including Pdgfb, Igfbp5, Flrt2, and Cxcl12, among which FLRT2 (p <0.01) and CXCL12 (p <0.001) activated hepatic stellate cells in vitro. scRNA-seq revealed replacement of zonated LSEC subpopulations with capillarized, proliferative, sprouting and secretory endothelial cell subsets that promote liver fibrogenesis and angiogenesis. This fundamental dysregulation of LSEC gene expression and differentiation was caused by changes in chromatin accessibility and transcription factor activity following loss of Maf. Notably, endothelial MAF expression was also significantly reduced in human cirrhotic livers (p <0.0001).
Conclusions
Hepatic endothelial c-Maf protects against metabolic dysfunction-associated steatohepatitis-like liver fibrosis and regulates endothelial differentiation and zonation by controlling chromatin opening.
Impact and implications
This work builds on the known importance of liver sinusoidal endothelial cells in liver function and disease. Here, transcription factor c-Maf is identified as a master regulator in maintaining normal differentiation and zonation of liver sinusoidal endothelial cells, thereby protecting against the development of liver fibrosis/cirrhosis. The findings are significant for researchers and clinicians focusing on liver disease, as they suggest potential new targets for therapeutic intervention. These findings could instruct the development of novel preventive treatment options and antifibrotic therapy regimens as well as liver repair strategies, benefiting patients, clinicians and policy makers in the management of liver disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


