有机催化和有机电合成作为姜酮制备的替代方法
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究提出了一种有机催化与有机电合成相结合的新型高效合成姜酮的方法。该工艺首先使用原位生成的三氟乙酸morpholinium有机催化剂将香兰素和丙酮进行醛醇缩合。这一步之后,通过使用石墨电极、KBr作为电解质和水作为质子源的简单设置,对所得到的烯酮中间体进行电化学还原。这种方法提供了一种简单而安全的方法来获取生姜酮,显著提高了反应效率。值得注意的是,该方法的简单性消除了对危险试剂和溶剂的需求,使其成为一种环保的替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
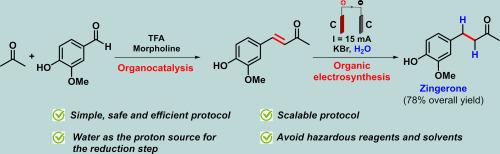
Organocatalysis and organic electrosynthesis as an alternative for zingerone preparation
This study presents a novel and efficient method for synthesizing zingerone through a combination of organocatalysis and organic electrosynthesis. The process begins with the aldol condensation of vanillin and acetone using in situ generated morpholinium trifluoroacetate organocatalyst. This step is followed by the electrochemical reduction of the resulting enone intermediate using a simple setup employing graphite electrodes, KBr as electrolyte and water as the proton source. This approach allows for a simple and safe strategy to access zingerone, significantly enhancing the reaction's efficiency. Notably, the method's simplicity eliminates the need for hazardous reagents and solvents, making it an environmentally friendly alternative.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


