杀菌剂氰唑胺合成的新方法
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
cyazofamid是一种高度选择性的杀菌剂,用于控制卵霉菌病原体,特别是马铃薯晚疫病的致病菌——疫霉。关键步骤是通过2-氨基-1-(对甲基)乙比1- 1与2,2,2-三氯乙酸乙酯环化来构建咪唑环。合成中的其他关键转化包括酰胺脱水成腈,区域选择性氯化和咪唑环的n -磺胺酰化。所有的反应都在制备规模上进行,总收率为28%。这种方法采用无害的、容易获得的试剂,并提供了改进的区域选择性,在氰唑胺的合成中提出了实际的进步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
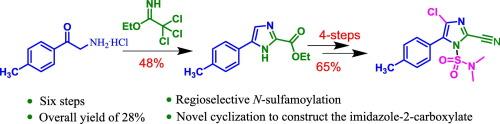
New approach to the synthesis of cyazofamid, fungicide
A robust and versatile six-step synthetic approach has been developed for the synthesis of cyazofamid, a highly selective fungicide used for controlling oomycete pathogens, particularly phytophthora infestans, the causing agent of late blight in potatoes. The pivotal step involves the construction of an imidazole ring via the cyclization of 2-amino-1-(p-tolyl)ethan-1-one with ethyl 2,2,2-trichloroacetimidate. Other key transformations in the synthesis include the dehydration of amide to nitrile, the regioselective chlorination, and N-sulfamoylation of the imidazole ring. All the reactions are performed on a preparative scale, affording an overall yield of 28 %. This approach employs non-hazardous, readily available reagents and offers improved regioselectivity, presenting a practical advancement in the synthesis of cyazofamid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


