不仅仅是一个漏水的肠道:肠道启动如何形成关节炎
IF 32.7
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
肠道微生物群形成了一个生态系统,为宿主提供了许多好处,如消化与营养生成,保护免受病原体和免疫系统成熟。与类风湿关节炎和脊椎关节炎相关的微生物生态系统的改变导致了肠道-关节假说,该假说认为这些生态变化导致免疫功能障碍,从而导致关节炎的发展。生态失调可能引发关节炎的机制包括分子模拟、粘膜免疫失调、微生物易位、免疫调节代谢物的产生和免疫细胞运输。我们讨论了支持这些机制的数据,并强调了知识上的误解、限制和差距。特别是,我们建议不要使用“漏肠”这个术语,因为肠道通透性和细菌易位对免疫系统的机制和影响是不同的。然而,类风湿关节炎和脊柱性关节炎可能是由这些疾病中个体亚群中独特的多种途径的聚合引起的。为了推动这一领域的发展,需要通过使用模式生物和干预性试验来单独和协调地考虑每种机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
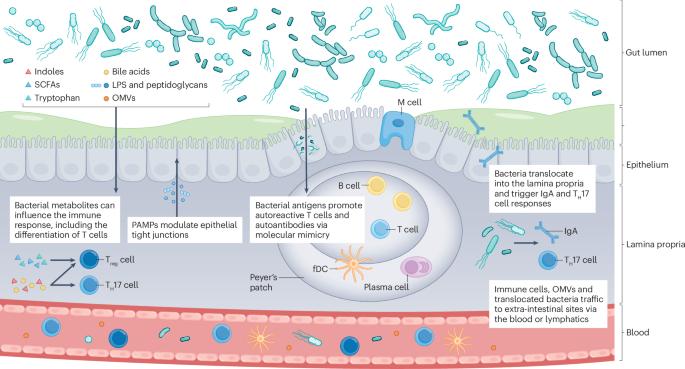
More than a leaky gut: how gut priming shapes arthritis
The gut microbiome forms an ecosystem that provides the host with numerous benefits such as digestion with nutrient generation, protection from pathogens and immune system maturation. Alterations in the microbial ecosystem associated with rheumatoid arthritis and spondyloarthritis have led to the gut–joint hypothesis, which postulates that these ecological changes cause immune dysfunction that contributes to the development of arthritis. Mechanisms by which dysbiosis might trigger arthritis include molecular mimicry, dysregulation of mucosal immunity, microbial translocation, production of immunomodulatory metabolites and immune cell trafficking. We discuss the data supporting each of these mechanisms, and highlight misconceptions, limitations and gaps in knowledge. In particular, we advise against the term ‘leaky-gut’ as the mechanisms and effects on the immune system of intestinal permeability and bacterial translocation are distinct. Nevertheless, rheumatoid arthritis and spondyloarthritis possibly result from the convergence of multiple pathways that could be unique to subgroups of individuals within these diseases. To move the field forward, each mechanism needs to be considered through the use of model organisms and interventional trials, individually and in concert. This Review discusses how alterations in the gut are linked to the development of arthritis. The authors challenge the concept of the ‘leaky gut’ hypothesis, stating that the effects of intestinal permeability and bacterial translocation on the immune system are distinct.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


