用于唾液中SARS-CoV-2基因组序列无标记检测的PNA-SERS生物传感器
IF 7.6
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
COVID-19大流行强调了快速、敏感和可获得的分子诊断的必要性。在这项研究中,我们提出了一种无标记的表面增强拉曼光谱(SERS)生物传感器,用于直接检测生物体液中的SARS-CoV-2 RNA。该传感器基于巯基化肽核酸(PNA)探针,该探针固定在沉积在功能化玻璃基板上的胶体金纳米颗粒(AuNPs)上。PNA分子的中性骨架、高序列亲和性和抗酶性等特性为其与靶序列的稳定和选择性杂交提供了有利条件。而AuNPs可以实现强信号增强和出色的再现性,而不需要复杂的纳米制造技术。总的来说,生物传感器的制造完全依赖于标准的实验室程序和市售试剂,使其具有成本效益和易于扩展。目标RNA的检测通过无标记SERS进行,负责扩增核碱基的振动指纹。通过主成分分析(PCA)和回归分析(PCR)进行多因素分析,进一步提高了光谱辨别能力和检测灵敏度。该传感器的检测限为110 pM,处于唾液SARS-CoV-2 RNA浓度的临床相关范围内。在缓冲液和人工唾液中评估了检测性能,证明了该平台用于真实生物样品的潜力。此外,该装置具有高选择性,可有效区分完全匹配、不匹配和随机序列。这项工作强调了PNA-SERS生物传感器在快速、无扩增的病毒RNA检测方面的潜力,并为传染病的即时诊断提供了一种有前途的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
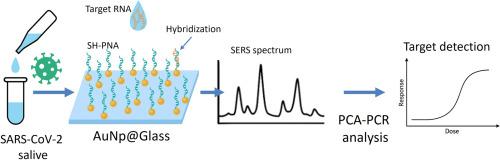
PNA-SERS biosensor for label-free detection of SARS-CoV-2 genomic sequences in saliva
The COVID-19 pandemic has emphasized the need for rapid, sensitive, and accessible molecular diagnostics. In this study, we present a label-free Surface-Enhanced Raman Spectroscopy (SERS) biosensor for the direct detection of SARS-CoV-2 RNA in biological fluids. The proposed sensor is based on a thiolated Peptide Nucleic Acid (PNA) probe immobilized on colloidal gold nanoparticles (AuNPs) deposited on functionalized glass substrates. A stable and selective hybridization with target sequences is provided by the intrinsic characteristics of PNA molecules, such as neutral backbone, high sequence affinity and enzymatic resistance. Whereas AuNPs enables strong signal enhancement and excellent reproducibility, without requiring complex nanofabrication techniques. Overall, the biosensor fabrication relies entirely on standard laboratory procedures and commercially available reagents, making it cost-effective and easily scalable. The detection of the target RNA occurs through label-free SERS, responsible for amplifying the vibrational fingerprint of nucleobases. Multivariate analysis through principal component analysis (PCA) and regression (PCR) further enhances spectral discrimination and detection sensitivity. The sensor exhibits a limit of detection of 110 pM, falling within the clinically relevant range of salivary SARS-CoV-2 RNA concentrations. Detection performance was assessed in both buffer and artificial saliva, demonstrating the potential of the platform for use with real biological samples. Moreover, the device demonstrates high selectivity, effectively distinguishing between fully matched, mismatched, and random sequences.
This work highlights the potential of PNA-SERS biosensors for rapid, amplification-free viral RNA detection and offers a promising approach for point-of-care diagnostics in infectious diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensors and Actuators Reports
Multiple-
CiteScore
9.60
自引率
0.00%
发文量
60
审稿时长
49 days
期刊介绍:
Sensors and Actuators Reports is a peer-reviewed open access journal launched out from the Sensors and Actuators journal family. Sensors and Actuators Reports is dedicated to publishing new and original works in the field of all type of sensors and actuators, including bio-, chemical-, physical-, and nano- sensors and actuators, which demonstrates significant progress beyond the current state of the art. The journal regularly publishes original research papers, reviews, and short communications.
For research papers and short communications, the journal aims to publish the new and original work supported by experimental results and as such purely theoretical works are not accepted.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


