短沟通:在合作繁殖的啮齿动物中,睾酮水平升高与先天免疫功能增强有关
IF 2.2
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology
Pub Date : 2025-07-28
DOI:10.1016/j.cbpa.2025.111911
引用次数: 0
摘要
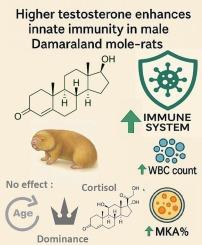
在许多脊椎动物中,睾酮水平升高被认为会损害免疫功能,这反映了繁殖和生存之间的权衡。然而,在合作繁殖者中,这种权衡可能会放松,因为社会冲突和性选择减少了。我们研究了圈养雄性达马拉兰鼹鼠(Fukomys damarensis)的睾酮、皮质醇和先天免疫之间的关系。达马拉兰鼹鼠是一种具有最小攻击性驱动的生殖竞争的社会性啮齿动物。利用微生物杀灭试验(mka)和白细胞计数作为免疫功能的代表,我们发现尿睾酮浓度与免疫强度呈正相关。尿中睾酮浓度较高的男性表现出更强的抗菌能力和更高的总白细胞计数。然而,尿睾酮与独立的免疫细胞分化无关。而生殖状态、年龄、体重和尿皮质醇浓度对任何免疫指标的影响有限。这些发现虽然具有相关性,但挑战了传统的内分泌-免疫权衡模型,并推断,在该物种中,睾酮可能是条件信号,而不是施加免疫抑制成本。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Short communication: Elevated testosterone correlates with enhanced innate immune function in a cooperatively breeding rodent
In many vertebrates, elevated testosterone is believed to compromise the immune function, reflecting a trade-off between reproduction and survival. However, such trade-offs may potentially be relaxed in cooperative breeders, where social conflict and sexual selection are reduced. We investigated the relationship between testosterone, cortisol, and innate immunity in captive male Damaraland mole-rats (Fukomys damarensis), a eusocial rodent with minimal aggression-driven reproductive competition. Using microbial killing assays (MKAs) and white blood cell counts as proxies of immune function, we found that urinary testosterone concentration was positively correlated with immune strength. Males with higher urinary testosterone concentrations exhibited significantly greater antimicrobial capacity and elevated total white blood cell counts. However, urinary testosterone was unrelated to independent immune cell differentials. While, reproductive status, age, body mass, and urinary cortisol concentrations had a limited effect on any immune metric. These findings, while correlative, challenge traditional endocrine-immune trade-off models and infer that, in this species, testosterone may signal condition rather than impose immunosuppressive costs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.00
自引率
4.30%
发文量
155
审稿时长
3 months
期刊介绍:
Part A: Molecular & Integrative Physiology of Comparative Biochemistry and Physiology. This journal covers molecular, cellular, integrative, and ecological physiology. Topics include bioenergetics, circulation, development, excretion, ion regulation, endocrinology, neurobiology, nutrition, respiration, and thermal biology. Study on regulatory mechanisms at any level of organization such as signal transduction and cellular interaction and control of behavior are also published.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


