炎症性肠病临床试验设计的最新创新
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
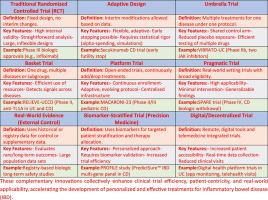
炎症性肠病(IBD)的临床试验设计正在不断发展,以应对药物开发和批准方面的挑战。对于临床开发,值得注意的创新包括贝叶斯设计、自适应设计、综合阶段试验和主方案(如伞式、篮子式和平台试验)。包括生物标志物驱动的策略和精准医学(PM)试验,旨在实现基于预后或预测标记的患者分层,利用分子特征来定制治疗。然而,最近的研究强调了这种方法的前景和复杂性。患者报告的结果(pro)作为关键终点已获得突出地位,使试验与以患者为中心的措施和强调症状和生活质量指标的监管指南保持一致。正在整合数字卫生工具和人工智能(AI),以简化试验进行,从远程监测和远程医疗就诊到人工智能辅助招聘和数据分析。实用试验和现实世界证据整合(RWE)旨在通过评估常规护理环境中的治疗来补充传统疗效试验。总之,这些创新标志着IBD临床试验设计的新时代,旨在加快治疗开发并增强试验与患者护理的相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Recent innovations in clinical trial design for inflammatory bowel disease
Clinical trial design in inflammatory bowel disease (IBD) is evolving to address challenges in drug development and approvals. For clinical development, notable innovations include Bayesian designs, adaptive designs, integrated-phase trials and master protocols (such as umbrella, basket, and platform trials). The inclusion of biomarker-driven strategies and precision medicine (PM) trials bring aim to enable patient stratification based on prognostic or predictive markers, leveraging molecular signatures to customize therapy. However, recent studies highlight both the promise and complexity of this approach. Patient-reported outcomes (PROs) have gained prominence as key endpoints, aligning trials with patient-centric measures and regulatory guidance that emphasize symptoms and quality-of-life metrics. Digital health tools and artificial intelligence (AI) are being integrated to streamline trial conduct, from remote monitoring and telemedicine visits to AI-assisted recruitment and data analysis. Pragmatic trials and the integration of real-world evidence (RWE) aim to complement traditional efficacy trials by evaluating treatments in routine care settings. Together, these innovations mark a new era in IBD clinical trial design, aiming to expedite therapeutic development and enhance the relevance of trials to patient care.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
2.50%
发文量
131
审稿时长
4-8 weeks
期刊介绍:
Current Opinion in Pharmacology (COPHAR) publishes authoritative, comprehensive, and systematic reviews. COPHAR helps specialists keep up to date with a clear and readable synthesis on current advances in pharmacology and drug discovery. Expert authors annotate the most interesting papers from the expanding volume of information published today, saving valuable time and giving the reader insight on areas of importance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


