形态和粘附双重仿生纳米疫苗通过亚细胞运输调节促进抗原交叉递呈
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
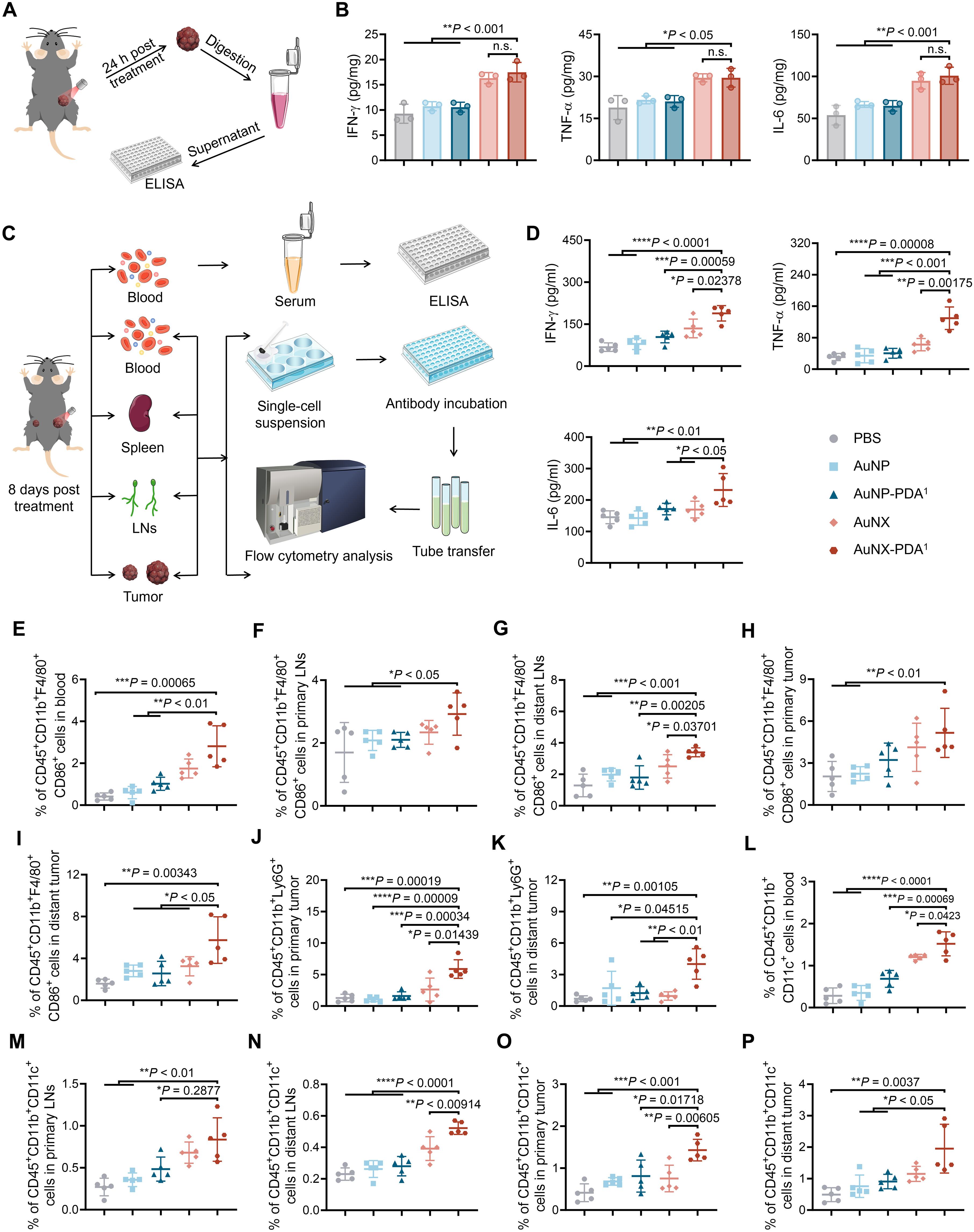
原位肿瘤疫苗具有巨大的免疫治疗潜力,因为它们产生完整的肿瘤源性抗原(tda)来激活抗肿瘤免疫反应。然而,释放的tda的快速降解和清除严重阻碍了随后的抗原呈递和原位疫苗的最终效果。在这里,我们合成了包被聚多巴胺(AuNX-PDA)的金纳米苍耳,分别模拟了苍耳和贻贝的形态和生物学粘附特性。AuNX-PDA促进近红外II光热处理后释放的TDAs的有效吸收,并将吸收的TDAs传递到树突状细胞的内质网和高尔基体进行交叉递呈,从而激活CD8 + T细胞,实现高效的肿瘤特异性免疫。纳米疫苗(NV)通过在B16F10黑色素瘤和4T1乳腺癌小鼠模型中产生强大的抗肿瘤免疫应答,显著抑制辐照原发肿瘤和未辐照远处肿瘤。这些发现强调了形态和粘附双重仿生NVs在全肿瘤疫苗治疗中的效力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Morphology– and adhesion–dual biomimetic nanovaccine boosts antigen cross-presentation through subcellular transport regulation
In situ tumor vaccines have immense potential for immunotherapy because they generate whole tumor–derived antigens (TDAs) to activate antitumor immune responses. However, the rapid degradation and clearance of the released TDAs severely hinder subsequent antigen presentation and the final efficacy of the in situ vaccine. Here, we synthesized gold nanoxanthium coated with polydopamine (AuNX-PDA) to mimic the morphological and biological adhesion properties of xanthium and mussels, respectively. AuNX-PDA facilitated effective absorption of released TDAs after near-infrared II photothermal treatment and delivery of the absorbed TDAs to the endoplasmic reticulum and Golgi apparatus of dendritic cells for cross-presentation, thereby activating CD8+ T cells for efficient tumor-specific immunity. The nanovaccine (NV) significantly inhibited irradiated primary tumors and nonirradiated distant tumors by producing robust antitumor immune responses in B16F10 melanoma and 4T1 breast cancer mouse models. These findings highlight the potency of morphology– and adhesion–dual biomimetic NVs in whole-tumor vaccine therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


