γδ T细胞受体对外来抗原的抗体样识别
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
与γδ T细胞相比,B细胞和αβ T细胞的抗原识别原理已经得到了很好的描述。γδT细胞通过其特异性赋予受体(γδ tcr)直接结合蛋白抗原。一种已知的γδ T细胞和B细胞模型抗原是藻红蛋白(PE),一种来自红藻和蓝藻的光收集蛋白。在这里,我们探讨了人γδTCR对PE的反应性,其中v δ 1v γ - 5 TCR直接结合诱导近端信号传导和细胞活化。我们测定了γδ tcr -植红蛋白免疫复合物的低温电镜(cryo-EM)结构。然后,我们确定了抗体片段和与PE结合的αβTCR的低温电镜结构。这揭示了聚类利用顶端芳香残基介导与共同PE表位的接触。对γδTCR的比较分析显示其具有多种抗体样特征,包括顶端芳香残基的富集。我们的研究结果进一步揭示了γδTCR对抗原识别的不同方面。本文章由计算机程序翻译,如有差异,请以英文原文为准。
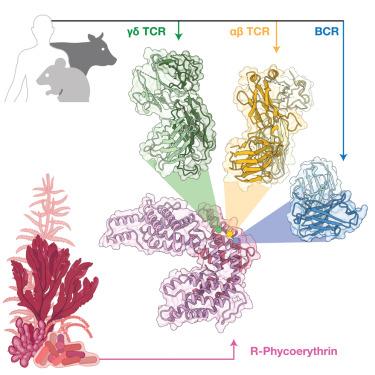
Antibody-like recognition of a γδ T cell receptor toward a foreign antigen
The antigen recognition principles of B cells and αβ T cells have been well described compared to those of the γδ T cell. By way of their specificity conferring receptor (γδTCR), γδ T cells can directly bind proteinaceous antigens. A known γδ T cell and B cell model antigen is phycoerythrin (PE), a light harvesting protein from rhodophytes and cyanobacteria. Here we probed human γδTCR reactivity to PE, in which a Vδ1Vγ5 TCR bound directly to induce proximal signaling and cellular activation. We determined the cryoelectron microscopy (cryo-EM) structure of the γδTCR-phycoerythrin immune complex. We then determined the cryo-EM structures of an antibody fragment and an αβTCR bound to PE. This revealed convergent use of apical aromatic residues to mediate contacts with a common PE epitope. Comparative analyses of the γδTCR revealed multiple antibody-like characteristics, including an enrichment of apical aromatic residues. Our findings reveal further distinct facets of antigen recognition by the γδTCR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


