呼吸道病毒感染唤醒肺部转移性乳腺癌细胞
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
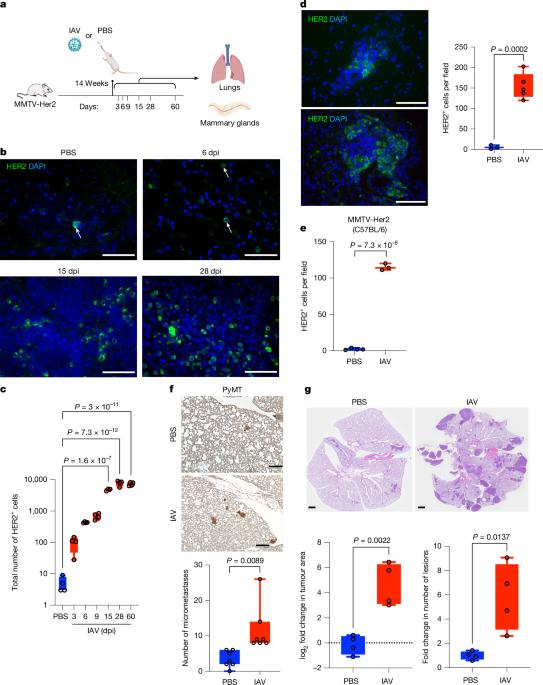
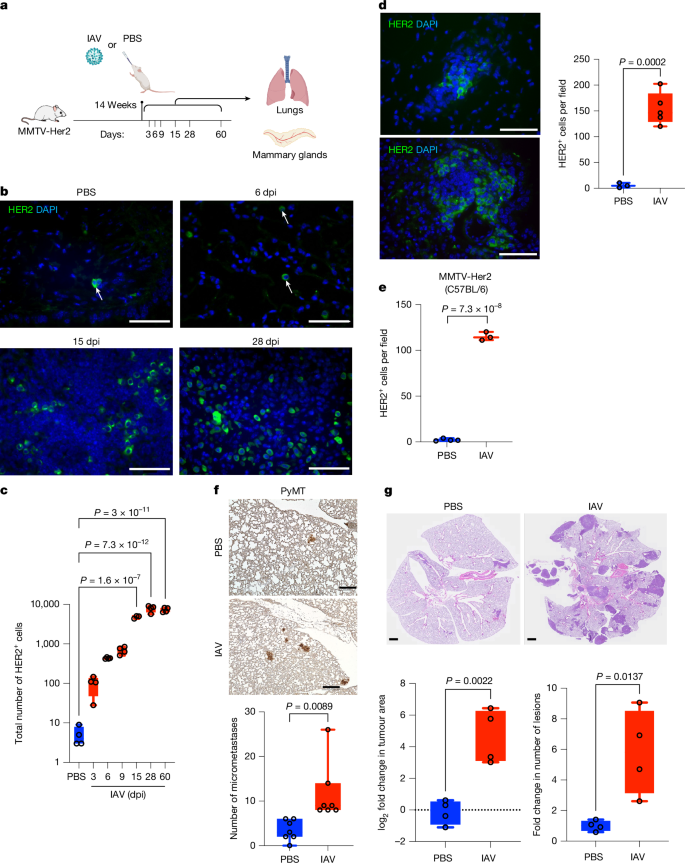
乳腺癌是全球第二大常见癌症,大多数死亡是由转移性疾病引起的,通常是在长期临床休眠之后1。了解破坏休眠播散性癌细胞(DCCs)休眠的机制对于解决转移进展至关重要。由流感和SARS-CoV-2等呼吸道病毒引起的感染会引发局部和全身炎症2,3。在这里,我们在小鼠中证明,流感和SARS-CoV-2感染导致肺部乳腺DCC中前休眠表型的丧失,导致DCC在感染后几天内增殖,并在两周内癌细胞大量扩增成转移灶。这些表型转变和扩增依赖于白细胞介素-6。我们发现,dcc损害肺T细胞活化,CD4+ T细胞在流感感染后通过抑制CD8+ T细胞活化和细胞毒性维持肺转移负荷。至关重要的是,这些实验结果与人类观测数据一致。对来自UK Biobank(所有癌症)和Flatiron Health(乳腺癌)数据库的癌症幸存者的分析显示,与未感染的癌症幸存者相比,SARS-CoV-2感染大大增加了癌症相关死亡率和肺转移的风险。这些发现强调了呼吸道病毒感染对转移性癌症复发的巨大影响,为传染病与癌症转移之间的联系提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Respiratory viral infections awaken metastatic breast cancer cells in lungs
Breast cancer is the second most common cancer globally, with most deaths caused by metastatic disease, often following long periods of clinical dormancy1. Understanding the mechanisms that disrupt the quiescence of dormant disseminated cancer cells (DCCs) is crucial for addressing metastatic progression. Infections caused by respiratory viruses such as influenza and SARS-CoV-2 trigger both local and systemic inflammation2,3. Here we demonstrate, in mice, that influenza and SARS-CoV-2 infections lead to loss of the pro-dormancy phenotype in breast DCCs in the lung, causing DCC proliferation within days of infection and a massive expansion of carcinoma cells into metastatic lesions within two weeks. These phenotypic transitions and expansions are interleukin-6 dependent. We show that DCCs impair lung T cell activation and that CD4+ T cells sustain the pulmonary metastatic burden after the influenza infection by inhibiting CD8+ T cell activation and cytotoxicity. Crucially, these experimental findings align with human observational data. Analyses of cancer survivors from the UK Biobank (all cancers) and Flatiron Health (breast cancer) databases reveal that SARS-CoV-2 infection substantially increases the risk of cancer-related mortality and lung metastasis compared with uninfected cancer survivors. These discoveries underscore the huge impact of respiratory viral infections on metastatic cancer resurgence, offering new insights into the connection between infectious diseases and cancer metastasis. Mouse models show that respiratory infections from viruses such as influenza and SARS-CoV-2 can trigger metastasis of dormant breast cancer cells in the lungs, a finding supported by epidemiological data from two large human cohorts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


