早期生命中aav载体成功递送HIV-1 bnab的决定因素
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
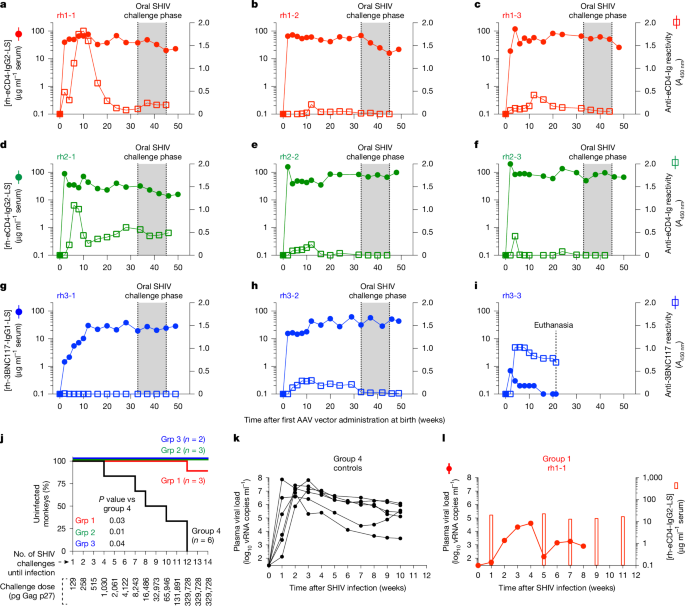
尽管在HIV-1预防方面取得了进展,但垂直传播在发展中国家仍然是一个紧迫的问题。鉴于广泛中和抗体(bNAbs)有望预防HIV-1,我们假设使用腺相关病毒(AAV)的bNAbs新生儿可以在婴儿期提供持久的HIV-1免疫。本研究以恒河猴(Macaca mulatta)为模型,研究人员发现,在出生时一次性注射编码bNAb 3BNC117的AAV载体可导致bNAb持续表达超过三年而无需重新注射。在模拟HIV-1通过母乳喂养和性交传播的粘膜攻击模型中,这种方法显著地保护了婴儿和青春期前的恒河猴免受猿人免疫缺陷病毒的感染。AAV-3BNC117给药时的年龄是成功的主要决定因素,并且与限制bNAb表达的宿主抗药物抗体的发生率呈负相关。与新生儿耐受原则一致3,4,在给药AAV-3BNC117后,新生恒河猴的bNAb表达水平高于年龄较大的婴儿和幼年恒河猴。此外,在子宫内暴露于重组3BNC117可抑制抗药物抗体,并改善aav载体在大龄婴儿中的bNAb递送。因此,我们的研究结果表明,新生儿和胎儿的免疫耐受可以用来改善灵长类动物出生后AAV对HIV-1 bNAbs的递送。由于恒河猴出生时一次性接种AAV载体可产生长达数年的HIV-1免疫,因此未来的研究应评估这一策略在预防围产期和青少年人类HIV-1感染方面的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Determinants of successful AAV-vectored delivery of HIV-1 bNAbs in early life
Despite advances in HIV-1 prophylaxis, vertical transmission remains a pressing problem in developing countries1. Given the promise of broadly neutralizing antibodies (bNAbs) for HIV-1 prevention2, we hypothesized that neonatal delivery of bNAbs using adeno-associated virus (AAV) could provide durable HIV-1 immunity during infancy. Here, using infant rhesus macaques (Macaca mulatta) as a model, we show that a one-time administration of an AAV vector encoding bNAb 3BNC117 at birth led to sustained bNAb expression for more than three years without redosing. This approach significantly protected both infant and pre-adolescent rhesus macaques from infection with simian–human immunodeficiency virus in mucosal challenge models that mimic HIV-1 transmission through breastfeeding and sexual intercourse. Age at the time of AAV-3BNC117 administration was a main determinant of success and was inversely correlated with the incidence of host anti-drug antibodies that restricted bNAb expression. Consistent with principles of neonatal tolerance3,4, newborn rhesus macaques exhibited higher levels of bNAb expression than older infants and juveniles following AAV-3BNC117 dosing. Furthermore, in utero exposure to recombinant 3BNC117 suppressed anti-drug antibodies and improved AAV-vectored delivery of this bNAb in older infants. Thus, our results suggest that neonatal and fetal immunological tolerance can be leveraged to improve postnatal AAV delivery of HIV-1 bNAbs in primates. Since years-long HIV-1 immunity can be generated in rhesus macaques from a one-time AAV vector administration at birth, future studies should evaluate the ability of this strategy to prevent perinatal and adolescent HIV-1 infections in humans. A single dose of an adeno-associated virus vector encoding an HIV-1 broadly neutralizing antibody given shortly after birth results in persistent antibody expression and protection from infection in rhesus macaque models of human HIV-1 transmission through breastfeeding and sexual intercourse.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


