从假设到预测:受试者内胃肠道生理变异性对生物等效性性别依赖性结果可能性的影响。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
在进行生物等效性(BE)研究时,由于各种原因,这是不常见的做法,因此有人呼吁将两性包括在内。在BE研究中,性别依赖结果的证实病例并不多;因此,在BE研究中纳入女性参与者被认为是公平和原则问题。大多数典型的BE研究是交叉设计,受试者之间的可变性(包括性别影响)不起作用。然而,在通过BE标准时,主体内可变性(WSV)仍然可以发挥重要作用。研究表明,胃肠道WSV以及药物配置因素传播到药代动力学中,决定了BE的预后。通过应用结肠运输时间(CTT)和胃排空时间(GET)的WSV测量中已知的基于性别的差异,我们说明了如何制定关于be研究中潜在的性别依赖结果的假设。这种方法支持在知情的情况下作出决定,决定是否有必要将女性参与者包括在内,或者是否有理由谨慎地放弃将她们纳入其中。进行虚拟生物等效性(VBE)评估(使用Simcyp V22中的VBE模块(Certara Predictive Technologies, Sheffield, UK)),其中三种具有不同溶出率的假设药物产品与多个虚拟测试配方对应进行评估。CTT和GET中性别依赖的WSV对VBE结果的影响通过对女性和男性受试者之间BE差异的系统分析来证明。这项研究为产生关于生理性别差异在BE结果中的传播的假设提供了路线图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
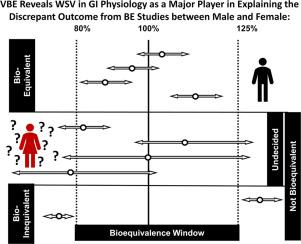
From hypothesis to forecasting: The impact of within-subject variability of gastro-intestinal physiology in relation to likelihood of sex-dependent outcome of bioequivalence
There are calls for inclusion of both sexes when conducting bioequivalence (BE) studies as this is not common practice for variety of reasons. There are not many proven cases for sex-dependent outcome of BE studies; hence inclusion of female participants in BE studies is considered as a matter of equity and principle. Most typical BE studies are cross-over design where between-subject variability (including sex effects) plays no role. However, within-subject variability (WSV) can still play a significant role, in passing the BE criteria.
It has been shown that WSV of gastrointestinal (GI) tract as well as drug disposition factors propagate to the pharmacokinetics, defining the outcome of BE. By applying known sex-based differences in WSV measures of colonic transit time (CTT) and gastric emptying time (GET), we illustrate how hypotheses can be formulated regarding potential sex-dependent outcomes in BE studies. This approach supports informed decision-making on the necessity of including female participants or justifying prudent waivers for their inclusion.
Virtual Bioequivalence (VBE) assessments were carried out (using VBE module in Simcyp V22 (Certara Predictive Technologies, Sheffield, UK)) where three hypothetical drugs product with distinct dissolution rates were evaluated against multiple virtual test formulation counterparts. The influence of sex-dependent WSV in CTT and GET on VBE outcomes was demonstrated by systematic analysis of discordance between BE in female vs. male subjects. The exercise provided a roadmap for generating hypotheses regarding the propagation of sex differences in physiology to BE outcome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


