通过体外、体内和网络药理综合分析,发现半魁地姆及其亲本种具有有效的抗骨关节炎药物作用
IF 6.2
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
骨关节炎(OA)的全球患病率不断上升,迫切需要更安全的替代现有治疗方法。摘要半毛丹巴(SC)及其两个亲本种Altingia chinensis (AC)和Liquidambar formosana (LF)传统上被用于治疗炎症,但在现代药理学中仍未得到充分的研究。本研究通过体外、体内和网络药理学相结合的方法来评估其抗骨关节炎的机制,以开发天然的抗骨关节炎药物。这三种植物提取物对lps刺激的Raw264.7巨噬细胞产生NO和ROS的抑制作用为7.8125 μg/mL (P <; 0.01)。LC-MS、网络药理学、分子对接、分子动力学模拟等手段鉴定出6个与PI3K/Akt/NF-κB通路相互作用的关键化合物(3,3′,4-三- o -甲基鞣花酸、半胱甘肽、没食子酸、油梨内酯、反式白藜芦醇3- o -β-葡萄糖苷和咖啡烯基酸)。值得注意的是,反式白藜芦醇3-O-β-葡萄糖苷对NO/ROS的抑制作用优于地塞米松。体内实验显示,所有提取物都能改善mia诱导的大鼠的细胞凋亡、蛋白聚糖损失和软骨降解,在不同物种之间的疗效相当(P >; 0.05)。具体而言,经组织学和免疫组织化学分析证实,所有提取物均可降低炎症标志物(i - κ b、IL-6、TNF-α、iNOS、p65)的表达,促进软骨修复。本研究首次提供了SC、AC和LF通过调节PI3K/Akt/NF-κB通路和抑制氧化应激作为有效的抗骨关节炎药物的药理学验证,反式白藜芦醇3-O-β-葡萄糖苷在这三个物种中被鉴定为一种高效的生物活性成分。它们的同等功效支持了AC和LF作为可持续替代品,减轻了SC的保护压力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
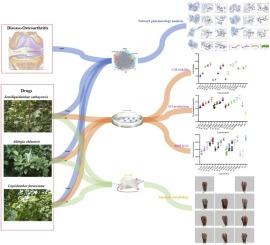
Discovery of Semiliquidambar cathayensis and its parent species as potent anti-osteoarthritic agents through integrated in vitro, in vivo and network pharmacological analyses
The increasing global prevalence of osteoarthritis (OA) underscores the urgent need for safer alternatives to existing therapies. Semiliquidambar cathayensis (SC) and its two parent species, Altingia chinensis (AC) and Liquidambar formosana (LF), have been traditionally employed to treat inflammatory conditions, yet they remain insufficiently studied in modern pharmacology. This study evaluated their anti-OA mechanisms through integrated in vitro, in vivo, and network pharmacology approaches to develop natural anti-osteoarthritic agents. The extracts of these three species potently inhibited NO and ROS production (P < 0.01) at 7.8125 μg/mL in LPS-stimulated Raw264.7 macrophages. LC-MS, network pharmacology, molecular docking, and molecular dynamics simulation identified six key compounds (3,3′,4-tri-O-methylellagic acid, galbacin, gallic acid, loliolide, trans-resveratrol 3-O-β-glucoside, and prenylcaffeate) interacting with the PI3K/Akt/NF-κB pathway from these three plants. Notably, trans-resveratrol 3-O-β-glucoside surpassed dexamethasone in inhibiting NO/ROS. In vivo assays revealed that all extracts ameliorated apoptosis, proteoglycan loss, and cartilage degradation in MIA-induced rats, with comparable efficacy (P > 0.05) among species. Specifically, all of the extracts reduced the expression of inflammatory markers (IκB, IL-6, TNF-α, iNOS, p65) and promoted cartilage repair, as confirmed by histological and immunohistochemical analysis. This study provides the first pharmacological validation of SC, AC, and LF as potent anti-osteoarthritic agents through modulation of the PI3K/Akt/NF-κB pathway and suppression of oxidative stress, with trans-resveratrol 3-O-β-glucoside identified as a highly potent bioactive component within these three species. Their equivalent efficacy supports AC and LF as sustainable substitutes, alleviating conservation pressure on SC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


