一锅法合成fe2o3负载的三氧化钨异质结用于快速和高度可回收的光催化硝基苯还原
IF 4.6
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
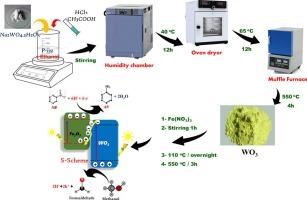
芳香族化合物由于其持久性和潜在的诱变作用,近年来作为一个重要的环境问题受到了人们的关注。为了解决这个问题,科学家们已经将光催化降解技术作为一个有前途的解决方案,特别是将有毒的硝基芳香族化合物转化为更安全的胺衍生物。在这项工作中,采用聚合物表面活性剂辅助生长的方法合成了WO3纳米结构,然后加入5.0-20.0 wt%的氧化铁(Fe2O3)纳米颗粒来开发可见光活性光催化剂。结构和光学分析表明,在WO3中掺入Fe2O3可以形成介孔结构,同时提高了其光学性能。值得注意的是,添加15wt % Fe2O3后,WO3纳米晶体的带隙(Eg)从3.0 eV大幅降低至2.54 eV,大大改善了可见光捕获。该光催化剂用于硝基苯(NB)在可见光下的高效光还原制苯胺(AN)。使用2.0 g/L剂量的15wt % Fe2O3-WO3, NB在40分钟内完全转化为AN,反应速率高达7.5 x 10-2 min−1,这归因于增强的电荷载流子迁移率和有效的电子空穴分离。此外,该催化剂表现出优异的可回收性,在5次再生循环后仍保持其初始效率的92.5%。机制研究表明,Fe2O3-WO3异质结促进了S-scheme电荷转移途径,优化了氧化还原电位,同时最小化了重组损失。这些发现突出了这种可持续光催化剂在工业规模化学合成中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
One-pot synthesis of Fe2O3-supported tungsten trioxide heterojunctions for rapid and highly recyclable photocatalytic nitrobenzene reduction
Aromatic compounds have recently gained attention as a significant environmental issue because of their persistent nature and potential mutagenic effects. To address this issue, scientists have turned to photocatalytic degradation techniques as a promising solution, particularly for converting toxic nitroaromatic compounds into safer amine derivatives. In this work, WO3 nanostructures were synthesized using a polymer surfactant-assisted growth method, followed by incorporating 5.0–20.0 wt% Iron(III) oxide (Fe2O3) nanoparticles to develop visible-light-active photocatalysts. Structural and optical analysis revealed that incorporating Fe2O3 into WO3 resulted in a mesoporous structure while enhancing its optical performance. Notably, adding 15 wt% Fe2O3 drastically reduced the bandgap (Eg) of WO3 nanocrystals from 3.0 eV to 2.54 eV, demonstrating greatly improved visible-light capture. This optimized photocatalyst was employed for the efficient photoreduction of nitrobenzene (NB) to aniline (AN) under visible light. Using a 2.0 g/L dose of 15 wt% Fe2O3-WO3, complete NB conversion to AN was achieved in just 40 min, with a high reaction rate of 7.5 x 10-2 min−1, attributed to enhanced charge carrier mobility and effective electron-hole separation. Additionally, the catalyst demonstrated excellent recyclability, maintaining 92.5 % of its initial efficiency after 5 regeneration cycles. Mechanistic studies revealed that the Fe2O3-WO3 heterojunction facilitates an S-scheme charge transfer pathway, optimizing redox potential while minimizing recombination losses. These findings highlight the potential of such sustainable photocatalysts for industrial-scale chemical synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Science and Engineering: B
工程技术-材料科学:综合
CiteScore
5.60
自引率
2.80%
发文量
481
审稿时长
3.5 months
期刊介绍:
The journal provides an international medium for the publication of theoretical and experimental studies and reviews related to the electronic, electrochemical, ionic, magnetic, optical, and biosensing properties of solid state materials in bulk, thin film and particulate forms. Papers dealing with synthesis, processing, characterization, structure, physical properties and computational aspects of nano-crystalline, crystalline, amorphous and glassy forms of ceramics, semiconductors, layered insertion compounds, low-dimensional compounds and systems, fast-ion conductors, polymers and dielectrics are viewed as suitable for publication. Articles focused on nano-structured aspects of these advanced solid-state materials will also be considered suitable.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


