海泡石对Li+, Na+和K+储存的分子尺度吸附机制和机械稳定性:来自分子动力学模拟的见解
IF 4.3
2区 材料科学
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
海泡石是一种水合硅酸镁,以其纤维结构而闻名,因其吸附多种金属离子的能力而广泛应用于水处理。海泡石的力学性能是海泡石在实际应用中至关重要的,但它的力学性能在很大程度上被忽视了。为了解决这一空白,本文采用分子动力学(MD)模拟来探索海泡石的力学特性,包括结构参数、体积模量和杨氏模量。研究结果表明,沸石水分子对提高海泡石的力学性能有积极作用。随着沸石水分子数的增加,在x和y方向上的杨氏模量值呈现明显的增大趋势。然而,沸石水分子的数量对z方向的杨氏模量和泊松比没有显著影响。此外,本文还评估了海泡石对Li+、Na+和K+的吸附能力。结果表明,随着离子浓度的升高,吸附在表面的离子的绝对数量增加,但相应的吸附率却明显下降。根据吸附量,确定海泡石对离子的选择性顺序为:Na+ >;李+比;K +。MD模拟阐明了碱金属离子与海泡石表面相互作用和粘附的吸附机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
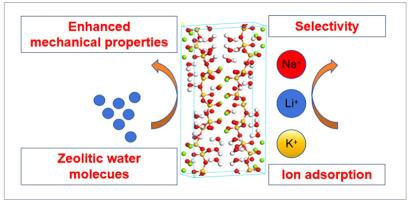
Molecular-scale adsorption mechanisms and mechanical stability of sepiolite for Li+, Na+ and K+ storage: Insights from molecular dynamics simulations
Sepiolite, a hydrated magnesium silicate known for its fibrous structure, is extensively utilized in water treatment for its ability to adsorb a variety of metal ions. Despite its widespread utilization, the mechanical properties of sepiolite, which are crucial for its practical applications, have been largely overlooked. To address this gap, this paper employs molecular dynamics (MD) simulation to explore the mechanical characteristics of sepiolite, including structural parameters, bulk modulus, and Young's modulus. The research findings reveal that zeolitic water molecules contribute positively to enhancing the mechanical properties of sepiolite. As the number of zeolitic water molecules rises, the Young's modulus values in the x and y directions show a clear increasing trend. However, the quantity of zeolitic water molecules does not significantly affect the Young's modulus in the z direction or the Poisson's ratio. Additionally, the paper assesses the adsorption capacity of sepiolite for Li+, Na+, and K+. The results indicate that as ion concentration rises, the absolute number of ions adsorbed onto the surface increases, yet the corresponding adsorption percentage shows a notable decline. Based on the adsorption capacity, the ion selectivity order of sepiolite is determined to be: Na+ > Li+ > K+. The MD simulations elucidate the adsorption mechanism by which alkali metal ions interact with and adhere to the sepiolite surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Particuology
工程技术-材料科学:综合
CiteScore
6.70
自引率
2.90%
发文量
1730
审稿时长
32 days
期刊介绍:
The word ‘particuology’ was coined to parallel the discipline for the science and technology of particles.
Particuology is an interdisciplinary journal that publishes frontier research articles and critical reviews on the discovery, formulation and engineering of particulate materials, processes and systems. It especially welcomes contributions utilising advanced theoretical, modelling and measurement methods to enable the discovery and creation of new particulate materials, and the manufacturing of functional particulate-based products, such as sensors.
Papers are handled by Thematic Editors who oversee contributions from specific subject fields. These fields are classified into: Particle Synthesis and Modification; Particle Characterization and Measurement; Granular Systems and Bulk Solids Technology; Fluidization and Particle-Fluid Systems; Aerosols; and Applications of Particle Technology.
Key topics concerning the creation and processing of particulates include:
-Modelling and simulation of particle formation, collective behaviour of particles and systems for particle production over a broad spectrum of length scales
-Mining of experimental data for particle synthesis and surface properties to facilitate the creation of new materials and processes
-Particle design and preparation including controlled response and sensing functionalities in formation, delivery systems and biological systems, etc.
-Experimental and computational methods for visualization and analysis of particulate system.
These topics are broadly relevant to the production of materials, pharmaceuticals and food, and to the conversion of energy resources to fuels and protection of the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


