通过与氨基试剂偶联的西尼地平的开创性分光光度分析:在药品和生物流体中的应用
IF 1.3
4区 化学
Q4 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
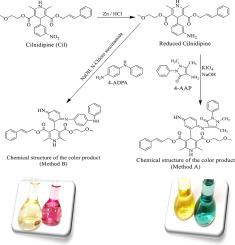
西尼地平是二氢吡啶类钙通道阻滞剂中重要的降压药物。药物制剂和生物液样品中西尼地平浓度的精确定量对确保治疗效果和安全性至关重要。测量西尼地平量的传统分析技术包括各种方法,氧化偶联反应被证明特别有效。本研究提出了一种利用4-氨基安替比林和4-氨基二苯胺氧化偶联分光光度法定量西尼地平的新方法。这些反应产生的有色化合物可以在528 nm和721 nm的可见光谱中检测到,显着提高了灵敏度和准确性。方法A和方法B在1-55µg/mL和1-30µg/mL的浓度范围内均符合比尔定律,方法A和方法B的摩尔吸光度分别为1.098×104 L/(mol⋅cm)和2.1179×104 L/(mol⋅cm),具有较高的分析鲁棒性。方法A和方法B的检出限分别为0.1159和0.3865µg/mL,定量限分别为0.1976和0.5848µg/mL,具有较强的分析性能。对线性度和精密度进行了彻底的验证,方法A和方法b的Sandell灵敏度分别为0.04486µg/cm2和0.02325µg/cm2。这种创新的方法为研究人员和医疗保健专业人员提供了一种可靠的工具,可以准确测量西尼地平,从而提高治疗效果并确保高标准的药品质量。两种方法均能有效测定西尼地平;药品片剂的平均回收率为99.53% ~ 100.2%,人尿和血清样品的平均回收率为99.77% ~ 100.58%,未发现商业剂型中存在共存添加剂的侵入。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pioneering spectrophotometric analysis of cilnidipine via coupling with amino reagents: application to pharmaceuticals and biological fluids
Cilnidipine is an important antihypertensive medication within the dihydropyridine class of calcium channel blockers. The precise quantification of cilnidipine concentrations in pharmaceutical formulations and biological fluid samples is crucial for ensuring therapeutic efficacy and safety. Traditional analytical techniques for measuring cilnidipine amounts have included various methods, with oxidative coupling reactions proving particularly effective. This study presents a novel spectrophotometric method for cilnidipine quantification, employing oxidative coupling with 4-aminoAntipyrine and 4-amino diphenylamine. These reactions yield colored compounds that can be detected in the visible spectrum at wavelengths of 528 nm and 721 nm, significantly improving both sensitivity and accuracy. Methods A and B adhere to Beer's law across specified concentration ranges of 1–55 and 1–30 µg/mL, respectively, demonstrating high molar absorptivity of 1.098×104 L/(mol⋅cm) for method A and 2.1179×104 L/(mol⋅cm) for method B, which highlights their analytical robustness. The limit of detections (LOD) was estimation and found to be 0.1159 and 0.3865 µg/mL for methods A and B, correspondingly, while the limit of quantifications (LOQ) was 0.1976 (method A) and 0.5848 µg/mL (method B), showcasing their strong analytical performance. A thorough validation of linearity and precision was performed, with Sandell's sensitivity assessed at 0.04486 µg/cm2 for method A and 0.02325 µg/cm2 for method B. This innovative approach provides researchers and healthcare professionals with a reliable tool for accurate cilnidipine measurement, thereby enhancing treatment outcomes and ensuring high standards of pharmaceutical quality. The two suggested techniques effectively determined Cilnidipine; with a decent average recovery in pharmaceutical tablets 99.53%–100.2 % and in human urine and serum samples of 99.77%–100.58 %, no intrusions of co-existing additives present in commercial dosage forms were noted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
25.00%
发文量
17223
审稿时长
35 days
期刊介绍:
Chinese Journal of Analytical Chemistry(CJAC) is an academic journal of analytical chemistry established in 1972 and sponsored by the Chinese Chemical Society and Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Its objectives are to report the original scientific research achievements and review the recent development of analytical chemistry in all areas. The journal sets up 5 columns including Research Papers, Research Notes, Experimental Technique and Instrument, Review and Progress and Summary Accounts. The journal published monthly in Chinese language. A detailed abstract, keywords and the titles of figures and tables are provided in English, except column of Summary Accounts. Prof. Wang Erkang, an outstanding analytical chemist, academician of Chinese Academy of Sciences & Third World Academy of Sciences, holds the post of the Editor-in-chief.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


