m6A RNA甲基化参与父亲镉暴露引起的肝毒性的跨代遗传
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
镉(Cd)是一种广泛存在的环境污染物,与各种不利的健康影响有关。然而,镉暴露对后代肝毒性的父系遗传跨代效应及其潜在机制仍未明确。为了解决这一问题,雄性小鼠(F0)在饮用水中添加100 mg/L氯化镉(CdCl2) 3 个月。然后,将F0只雄性小鼠与健康雌性小鼠交配,产生F1和F2后代。分别在6 周和6 个月时评估肝功能。在这里,我们发现祖先镉暴露导致F1雄性和F2小鼠的肝损伤,其特征是肝脏脂肪变性和葡萄糖稳态受损。这些结果表明,祖先接触镉可诱导镉致肝损伤的跨代遗传,且在雄性后代中更为深刻。此外,甲基化RNA免疫沉淀(MeRIP)测序分析在CdCl2处理3 个月和9 个月的小鼠肝组织中分别鉴定出285和734个差异甲基化表达基因。其中,抑制Irs1和Il6st导致CdCl2诱导的细胞毒性增强,表明Irs1和Il6st可能参与了CdCl2诱导的肝损伤。更重要的是,在F1雄性和F2小鼠中也观察到Irs1和Il6st RNA甲基化的改变,与表型一致。进一步分析表明,Irs1甲基化参与镉诱导肝毒性的跨代遗传,可能是由METTL3介导的。总的来说,这些发现揭示了RNA甲基化在不良反应跨代遗传中的新作用,并加深了对疾病病因学的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
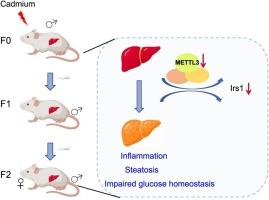
The involvement of m6A RNA methylation in the transgenerational inheritance of hepatotoxicity induced by paternal cadmium exposure
Cadmium (Cd), a widespread environmental pollutant, has linked with various adverse health effects. However, the paternally inherited transgenerational effects of cadmium exposure on hepatotoxicity in offspring and the underlying mechanism remained undefined. To address this issue, male mice (F0) were administrated with 100 mg/L cadmium chloride (CdCl2) in drinking water for 3 months. Then, F0 males were mated with healthy female mice to produce F1 and F2 offspring. Liver function was assessed at age of 6 weeks and 6 months, respectively. Herein, we showed ancestral cadmium exposure led to liver injury in F1 male and F2 mice, characterized by hepatic steatosis and impaired glucose homeostasis. These results suggest that ancestral exposure to cadmium could induce the transgenerational inheritance of cadmium-induced liver injury, which was more profound in male offspring. Furthermore, methylated RNA immunoprecipitation (MeRIP) sequencing analysis identified 285 and 734 differentially methylation expressed genes in mice liver tissue treated with CdCl2 for 3 months and 9 months, respectively. Among them, suppression of Irs1 and Il6st led to enhanced cytotoxicity induced by CdCl2, indicating that Irs1 and Il6st might be involved in CdCl2-induced liver injury. More importantly, the alteration of Irs1 and Il6st RNA methylation was also observed in F1 males and F2 mice, consistent with the phenotypes. Further analysis suggested the involvement of Irs1 methylation in the transgenerational inheritance of cadmium-induced hepatotoxicity, possibly mediated by METTL3. Collectively, these findings uncover the novel role of RNA methylation in the transgenerational inheritance of adverse effects and deepen the understanding of the etiology of disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


