GRAS优于遗憾:肺制剂中的赋形剂毒性。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-24
DOI:10.1016/j.ejpb.2025.114814
引用次数: 0
摘要
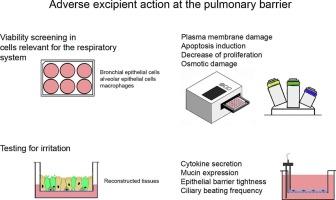
与其他给药途径类似,肺制剂通常含有赋形剂。与其他给药途径相比,可用于肺制剂的物质种类有限,其中大多数具有通常被认为是安全的(GRAS)状态。新的赋形剂必须经过体外和完整的体内测试才能获得监管机构的批准。总结了最常见的肺制剂(干粉吸入器、加压计量吸入器和雾化器)中所含辅料引起的毒性作用,并将其与批准浓度进行了比较。假设浓度远高于批准的浓度可以作为化合物安全性的指标。虽然许多赋形剂的细胞毒性浓度高于批准的浓度,但有些赋形剂的细胞毒性浓度却相反。对于这些化合物中的大多数,长期给药或在特定人群中报告了不良的肺效应。这表明低于细胞毒性阈值的浓度不太可能引起不良的呼吸影响。联合使用赋形剂会增加毒性作用,但很少有研究解决这个问题。由于细胞毒性试验和动物试验都不能可靠地确定辅料的毒性浓度,因此提出了额外的体外试验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Better GRAS than sorry: Excipient toxicity in pulmonary formulations
Similar to other routes of administration, pulmonary formulations typically contain excipients. The panel of substances that can be used in pulmonary formulations is limited compared to other routes of administration, and most of them have the generally regarded as safe (GRAS) status. New excipients must undergo in vitro and complete in vivo testing to be approved by regulatory authorities. The toxic effects induced by excipients contained in the most common pulmonary formulations (dry powder inhalers, pressurized metered dose inhalers, and nebulizers) were summarized to compare the cytotoxic effects to the approved concentrations. The hypothesis was that a concentration much higher than the approved one could serve as an indicator of the safety of the compound. While the cytotoxic concentration of many excipients was higher than the approved concentration, the opposite was found for some of them. For most of these compounds, adverse lung effects were reported with long-term administration or in specific populations. This suggests that concentrations lower than the cytotoxic threshold are unlikely to cause adverse respiratory effects. Combining excipients can increase toxic effects, but few studies address this. Since neither cytotoxicity testing nor animal testing can reliably identify toxic concentrations of excipients, additional in vitro tests have been proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


