自身免疫性和炎症性风湿病中的动态巨噬细胞表型
IF 32.7
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
巨噬细胞调节多种自身免疫性和炎性风湿病的炎症和纤维化过程。它们是高度可塑性的细胞,在与其他细胞、环境因素和细胞因子信号的动态相互作用下,在促炎、抗炎或促纤维化表型范围内发生变化。术语“M1巨噬细胞”,或经典活化,和“M2巨噬细胞”,或交替活化,以前分别用于表示促炎性和抗炎性巨噬细胞亚群,但这种分类系统已经过时,体内证据表明巨噬细胞表型连续体包括M1样,M2样和杂交表型。在类风湿关节炎、银屑病关节炎、系统性硬化症、系统性红斑狼疮、风湿性多肌痛和巨细胞动脉炎的滑膜炎和大血管炎的进展过程中,揭示驱动巨噬细胞可塑性和功能的具体机制,可以提高我们对疾病病理生理学的认识。类风湿关节炎的滑膜组织、系统性硬化症的纤维化皮肤和肺、系统性红斑狼疮的受损肾脏以及风湿性多肌痛和巨细胞动脉炎的法氏囊组织或大血管中的巨噬细胞可塑性增强。复杂的转录组学分析揭示了受影响器官活检中巨噬细胞的各种表型集群。此外,巨噬细胞的可塑性似乎是一些用于治疗上述疾病的标准化药物的目标。本文章由计算机程序翻译,如有差异,请以英文原文为准。

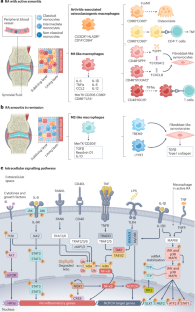
Dynamic macrophage phenotypes in autoimmune and inflammatory rheumatic diseases
Macrophages regulate inflammatory and fibrotic processes in several autoimmune and inflammatory rheumatic diseases. They are highly plastic cells, shifting within a range of pro-inflammatory and anti-inflammatory or pro-fibrotic phenotypes in response to dynamic interactions with other cells, environmental factors and cytokine signatures. The terms ‘M1 macrophages’, or classically activated, and ‘M2 macrophages’, or alternatively activated, were previously used to denote pro- and anti-inflammatory macrophage subsets, respectively, but this classification system has been outdated by in vivo evidence of a continuum of macrophage phenotypes that includes M1-like, M2-like and hybrid phenotypes. Deciphering the specific mechanisms that drive macrophage plasticity and function during the progression of rheumatoid arthritis, psoriatic arthritis, systemic sclerosis, systemic lupus erythematosus, and in synovitis and large vessel vasculitis in polymyalgia rheumatica and giant-cell arteritis, can improve our understanding of disease pathophysiology. Macrophage plasticity is enhanced in synovial tissue in rheumatoid arthritis, fibrotic skin and lung in systemic sclerosis, damaged kidney in systemic lupus erythematosus, and the bursal tissues or large vessels in polymyalgia rheumatica and giant-cell arteritis. Sophisticated transcriptomic analyses have revealed various phenotypic clusters of macrophages in biopsies of affected organs. Moreover, macrophage plasticity seems to be targeted by some standardized drugs used to treat the aforementioned conditions. Phenotypic and functional plasticity of macrophages is implicated in the pathophysiology of numerous autoimmune rheumatic diseases. In this Review, the authors discuss the latest insights into this complex and dynamic process and the potential implications for the treatment of these diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


