铱催化末端烯烯与丙二烯酰胺的区域选择性烷基化反应
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种由阳离子铱催化剂和酸性催化剂组成的协同催化剂对末端烯烯与丙二烯酰胺的区域选择性氢烷基化反应。氢烷基化以高度原子经济的方式进行。氢烷基化产物可通过脱氨基羰基化转化为γ,δ-不饱和酰胺。对反应机理的多次实验表明,三氟乙酸与烯丙烯加成的线性烯丙烯酯可能是该反应的中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
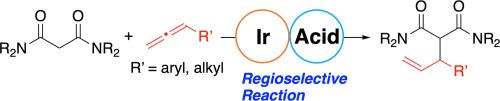
Iridium-catalyzed regioselective hydroalkylation of terminal Allenes with malonic amides
We report the regioselective hydroalkylation of terminal allenes with malonic amides by a cooperative catalyst consisting of a cationic iridium catalyst and an acid catalyst. The hydroalkylation proceeded in a highly atom-economical manner. The hydroalkylated product can be converted to a γ,δ-unsaturated amide through deaminocarbonylation. Several experiments on the reaction mechanism indicated that the linear allylic ester formed by the addition of trifluoroacetic acid to allene is a possible intermediate in the reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


