水基质中二苯甲酮-3的声溶降解:反应机理、转化产物、生态毒理学意义和微塑性相互作用
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
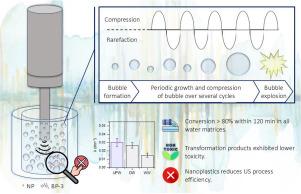
研究了低频超声对紫外滤光剂二苯甲酮-3 (BP-3)在不同水溶液中的降解效果。BP-3的超声降解遵循准一级动力学,在超纯水(UPW)中,使用20 kHz 71 W L−1的超声喇叭,在120分钟内实现了500 μg L−1的BP-3去除率>; 97%。初始pH从3变化到6和9对工艺效果的影响很小,相应的动力学常数速率分别为0.029、0.030和0.041 min−1。在不同水基质中进行的实验表明,表观速率常数从超纯水中的0.030 min−1降低到饮用水中的0.027 min−1和二级出水中的0.015 min−1。同样,250 mg L−1氯化物或10 mg L−1腐植酸的存在将降解率分别降低到0.016和0.020 min−1,而250 mg L−1碳酸氢的存在没有显著影响。纳米或微塑料的存在导致BP-3去除的适度减少,特别是较小的颗粒。电子顺磁共振(EPR)谱证实,在塑料的存在下,羟基自由基较少。UHPLC-TOF/MS鉴定了12个转化产物,主要由羟基化、去甲基化和环切割产生。根据ECOSAR软件的生态毒性分析,大多数转化产物的毒性低于母体化合物,增强了该工艺的环境可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sonolytic degradation of benzophenone-3 in water matrices: Reaction mechanism, transformation products, ecotoxicological implications & microplastic interaction
The degradation of the UV filter benzophenone-3 (BP-3) by low-frequency ultrasound was investigated in different aqueous matrices. BP-3 sonodegradation followed pseudo-first-order kinetics, achieving >97 % removal of 500 μg L−1 of BP-3 within 120 min in ultrapure water (UPW), using a 20 kHz ultrasound horn at 71 W L−1. Varying the initial pH from 3 to 6 and 9 had only a slight effect on the process efficacy, with corresponding kinetic constant rates of 0.029, 0.030, and 0.041 min−1, respectively. Experiments conducted in different water matrices showed a decrease in the apparent rate constant from 0.030 min−1 in ultrapure water to 0.027 min−1 in drinking water and 0.015 min−1 in secondary effluent. Similarly, the presence of 250 mg L−1 of chlorides or 10 mg L−1 of humic acid reduced the degradation rate to 0.016 and 0.020 min−1, respectively, while 250 mg L−1 of hydrogen carbonate had no significant effect. The presence of nano- or microplastics led to a moderate decrease in BP-3 removal, particularly with smaller particles. Electron paramagnetic resonance (EPR) spectroscopy confirmed that fewer hydroxyl radicals were available in the presence of plastics. Twelve transformation products were identified by UHPLC-TOF/MS, resulting mainly from hydroxylation, demethylation, and ring-cleavage. According to the ecotoxicity analysis using the ECOSAR software, most of the transformation products were less toxic than the parent compound, enhancing the environmental feasibility of the process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


