肾上腺素能信号作为自身免疫的治疗靶点
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
多发性硬化症(MS)是一种以自身免疫反应为特征的中枢神经系统(CNS)炎症性脱髓鞘疾病,其中t淋巴细胞和促炎白介素17A (IL-17A)都与疾病的发病机制有关。我们最近发现了TH17淋巴细胞极化中涉及β -肾上腺素能1和2受体(β1/2)的分子机制。联合而非单独对这些受体进行药理学和遗传抑制会损害t淋巴细胞产生IL-17A的能力,反而会促进分泌抗炎白介素-10 (IL-10)的保护性Treg细胞的分化。然而,目前尚不清楚这种调节机制是否可以作为一种新的治疗方法,用于由il - 17a产生的t淋巴细胞介导的自身免疫性疾病,如MS。使用MS的动物模型,称为实验性自身免疫性脑脊髓炎(EAE),我们研究了β肾上腺素能受体阻断(遗传和药理学)对EAE疾病进展、严重程度和TH17/Treg平衡的影响。遗传缺失β1/2受体,无论是全身性的还是特异性的t淋巴细胞,都能显著减轻EAE疾病的严重程度和动物的体重减轻。用普萘洛尔(亲脂)或纳多洛尔(含水)阻断β1/2受体的治疗性药理作用限制了与遗传模型相似的疾病严重程度和体重减轻,与抗il - 17a抗体联合治疗显示出最大的疾病缓解。所有模型均显示不同程度的TH17/Treg平衡移位和t淋巴细胞IL-17A生成减少。我们的数据描述了β1/2肾上腺素能信号在EAE中控制TH17/Treg细胞中的新作用,为疾病进展提供了新的见解,并为il - 17a相关自身免疫性疾病提供了潜在的新药物治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
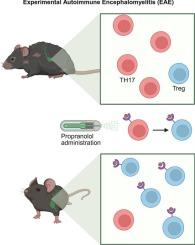
Beta adrenergic signaling as a therapeutic target for autoimmunity
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS) characterized by an autoimmune response where both T-lymphocytes and proinflammatory interleukin 17A (IL-17A) are implicated in the pathogenesis of the disease. We recently identified a molecular mechanism involving beta-adrenergic 1 and 2 receptors (β1/2) in the polarization of TH17 lymphocytes. Pharmacological and genetic inhibition of these receptors in combination, but not separately, impaired the ability of T-lymphocytes to produce IL-17A and instead promoted the differentiation of protective Treg cells that secrete anti-inflammatory interleukin-10 (IL-10). However, it remained unclear whether this regulatory mechanism could serve as a novel therapeutic approach for autoimmune disorders mediated by IL-17A-producing T-lymphocytes, like MS. Using an animal model of MS, termed experimental autoimmune encephalomyelitis (EAE), we addressed the impact of beta adrenergic receptor blockade (genetically and pharmacologically) on EAE disease progression, severity, and TH17/Treg balance. Genetic deletion β1/2 receptors, either systemically or specifically on T-lymphocytes, significantly attenuated EAE disease severity and animal weight loss. Therapeutic pharmacological blockade of β1/2 receptors with either propranolol (lipophilic) or nadolol (aqueous) limited disease severity and weight loss similar to the genetic models, with combination therapy with anti-IL-17A antibodies showing the greatest disease remission. All models showed degrees of shifted TH17/Treg balance and decreased T-lymphocyte IL-17A production. Our data depict a novel role for β1/2 adrenergic signaling in the control of TH17/Treg cells in EAE, and provide new insight into the disease progression as well as offer a potential new pharmacological therapy for IL-17A-related autoimmune diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


