具有肝糖异生抑制活性的casane二萜类化合物
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对Caesalpinia decapetala (Roth) Alston种子的化学研究导致了15种cassane二萜的分离,包括10种以前未描述的化合物(1-10)和5种已知的类似物(11-15)。1-10的结构通过1H, 13C, 2D NMR, HRESIMS和IR数据确定。通过比较实验电子圆二色性数据与计算值,并结合单晶x射线衍射分析,确定了它们的绝对构型。在结构上,化合物1和2是罕见的cassane二萜,在C-3上具有酮羰基。肝脏糖异生过度在2型糖尿病的发生发展中起重要作用。在本研究中,化合物1、3、4、6、11和12在20 μM浓度下对小鼠原代肝细胞糖异生表现出较强的抑制作用,抑制率分别为68.2±6.8%、78.4±3.5%、53.8±6.1%、94.8±5.5%、85.1±4.1%和106.9±2.9%。进一步的研究表明,这些化合物在1 ~ 50 μM的浓度范围内以剂量依赖的方式抑制糖异生。值得注意的是,化合物6和12在浓度为2 μM时表现出较强的糖异生抑制作用,与500 μM二甲双胍的效果相当,表明它们具有改善高血糖的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
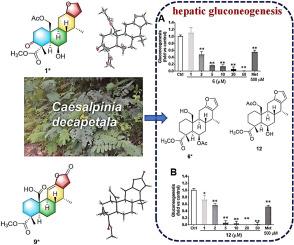
Cassane diterpenoids with hepatic gluconeogenesis inhibitory activity from the seeds of Caesalpinia decapetala (Roth) Alston
A chemical investigation of the seeds of Caesalpinia decapetala (Roth) Alston led to the isolation of fifteen cassane diterpenoids, including ten previously undescribed compounds (1–10) and five known analogues (11–15). The structures of 1–10 were determined using 1H, 13C, and 2D NMR, HRESIMS, and IR data. Their absolute configurations were confirmed by comparing experimental electronic circular dichroism data with calculated values, coupled with single-crystal X-ray diffraction analysis. Structurally, compounds 1 and 2 are rare cassane diterpenoids featuring a ketone carbonyl group at C-3. Excessive hepatic gluconeogenesis plays an important role in the occurrence and development of type 2 diabetes mellitus. In the present study, compounds 1, 3, 4, 6, 11, and 12 exhibited potent inhibitory effects on gluconeogenesis at a concentration of 20 μM in primary mouse hepatocytes, with inhibitory capacities of 68.2 ± 6.8 %, 78.4 ± 3.5 %, 53.8 ± 6.1 %, 94.8 ± 5.5 %, 85.1 ± 4.1 %, and 106.9 ± 2.9 %, respectively. Further studies indicated that these compounds inhibited gluconeogenesis in a dose-dependent manner across a concentration range from 1 to 50 μM. Notably, compounds 6 and 12 exhibited potent inhibitory effects on gluconeogenesis at a concentration of 2 μM, comparable to the effects of 500 μM metformin, suggesting their potential for ameliorating hyperglycemia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


