β-环糊精衍生物与氨氯地平包合物增强溶解度、药物释放和抗癌活性的比较研究
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
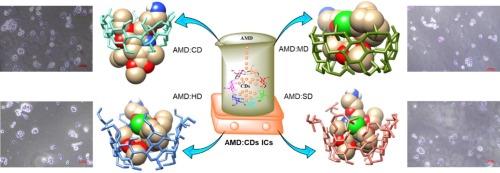
氨氯地平(AMD)是一种钙通道阻滞剂,由于其生物学特性而成为一种可行的抗癌药物。然而,其水溶性差和生物利用度低,阻碍了口服时的生理活性和治疗应用。本研究制备了AMD与纯环糊精(CDs)和三种不同的环糊精衍生物羟丙基β-环糊精(HD)、甲基β-环糊精(MD)和磺基丁醚-β-环糊精(SD)的包合物(ic),并比较了它们的理化和生物学性能。采用紫外可见光谱和荧光光谱测量了AMD:CD IC在水介质中的溶解度,并用Benesi-Hildebrand方法计算了其结合常数。此外,相溶解度研究证实了1:1 ic的形成,遵循al型剖面。在各种CD衍生物中,AMD:SD表现出较高的表观稳定常数(K1:1),为1447.5 M−1,表明SD与AMD具有较强的亲和力。采用共沉淀法制备了AMD:CDs (1:1) ic,并对其官能团、结晶度、形态变化和热稳定性进行了表征,表明AMD成功封装在CDs中。此外,分子对接研究证实了AMD被封装在CDs内,具有良好的结合能和稳定的相互作用。药物释放研究显示,在最初的爆发释放后,20分钟后持续释放,AMD:CDs的释放率在82 - 98%之间。最后,通过WST-1检测,与纯AMD和CDs相比,AMD:CDs ic在HCT-116细胞中表现出更好的细胞活力和细胞摄取。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comparative study of β-cyclodextrin derivatives with amlodipine inclusion complexes for enhanced solubility, drug release, and anticancer activity
Amlodipine (AMD), a calcium channel blocker, has become a viable anticancer treatment because of its biological properties. However, its poor water solubility and low bioavailability hinder its physiological activities and therapeutic applications when administered orally. In this study, inclusion complexes (ICs) of AMD with pure cyclodextrins (CDs) and three different CD derivatives, namely hydroxypropyl β-cyclodextrin (HD), methyl-β-cyclodextrin (MD), and sulfobutylether-β-cyclodextrin (SD), were prepared, and their physicochemical and biological properties were compared. The enhanced solubility of AMD:CD IC formation in aqueous media was measured using UV–Vis and fluorescence spectroscopy, and the binding constants were calculated using the Benesi-Hildebrand method. In addition, phase solubility studies confirmed the formation of 1:1 ICs, which followed an AL-type profile. Among the various CD derivatives, AMD:SD exhibited a high apparent stability constant (K1:1) of 1447.5 M−1, indicating a strong affinity between SD and AMD. The AMD:CDs (1:1) ICs were prepared using the co-precipitation method and characterized to identify the functional groups, crystallinity, morphological changes, and thermal stability, which indicated the successful encapsulation of AMD within CDs. Moreover, molecular docking studies confirmed the encapsulation of AMD within CDs with favorable binding energy and stable interactions. Drug release studies showed an initial burst release followed by a sustained release after 20 min, and the release percentage for the AMD:CDs was between 82 and 98 %. Finally, the AMD:CDs ICs exhibited superior cell viability and cellular uptake in HCT-116 cells using the WST-1 assay compared to that of pure AMD and CDs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


