胶体纳米银的细胞相互作用和海藻酸盐封盖在防止可溶性银离子浸出中的作用
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
纳米银的生物效应包括抗菌能力是众所周知的。纳米银,特别是具有生物聚合物作为封盖剂的小银颗粒,被认为与细胞和生物系统更相容。假说认为,大尺寸的生物聚合物可以防止银+离子的浸出,而银+离子是银nps毒性的主要原因。为了证实这一点,我们合成了海藻酸盐溶液还原和封盖的胶体银溶液,并进行了银离子释放动力学研究。总的来说,机械搅拌有助于离子释放过程,在前30分钟内离子释放量最大(1.8±1 ppm),而在非摇动条件下,在相同的持续时间内,离子释放量仅为0.19±0.6 ppm。30分钟后,离子释放可以忽略不计,与搅拌无关。有趣的是,Ag+离子的最大释放量仅为总释放量的5.6%。此外,胶体银被检测抗氧化活性,其惊人的高于标准抗坏血酸溶液。测定了银颗粒存在时SIF和SSF中两种关键消化酶胃蛋白酶和α-淀粉酶的活性。胃蛋白酶未受影响,但α-淀粉酶的活性随着颗粒浓度的增加而降低(p <;0.05)。接下来,我们研究了海藻酸盐覆盖的纳米银对六种主要分布在伤口部位和一组哺乳动物细胞上的细菌菌株的生物学效应。微生物的反应是剂量和时间依赖的。而纳米银对hscs、A-431、HaCaT、HEK-293、HeLa和THP-1的IC50分别为13.22、5.96、6.289、12.74、6.0、5.6 ppm。最后,通过对雌性BalB/小鼠静脉注射颗粒和图像分析,我们能够对颗粒对小鼠器官的安全性进行概述。本文章由计算机程序翻译,如有差异,请以英文原文为准。
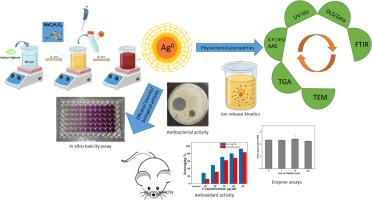
Cellular interactions of colloidal nanosilver and role of alginate capping in prevention of soluble Ag+ leaching
Biological effects including antimicrobial potencies of nanosilver are well known. Nanosilver particularly, small silver particles having a biopolymer as capping agent have been suggested to be more compatible to cells and biological system. Hypothesis says that a biopolymer, being large in size, could prevent the leaching of Ag+ ions, which are the primary cause of AgNPs toxicity. In order to corroborate this, we synthesized colloidal silver solution which was both reduced and capped by alginate solution and Ag+ ion release kinetics was performed. Overall, it was noted that mechanical agitation aids in the process of ion release which was maximum within first 30 min. (1.8 ± 1 ppm) whereas, only 0.19 ± 0.6 ppm release was observed in non-shaking conditions, in the same duration. After 30 min, the ion release was negligible, irrespective of agitation. Interestingly, the maximum amount of Ag+ ion released was only 5.6 % of total. Further, the colloidal silver was examined for antioxidant activity which was surprisingly higher than the standard ascorbic acid solution. Activity of two key digestive enzymes pepsin and α-amylase was assessed in presence of silver particles in SIF and SSF, respectively. Pepsin was unaffected but α-amylase showed reduced activity with increasing particle concentration (p < 0.05). Next, we examined the biological effects of alginate-capped nanosilver on six bacterial strains that predominantly populate wound sites and a panel of mammalian cells. Response of microbes was both dose- and time-dependent. Among tested, P. mirabilis and K. pneumoniae were able to revive themselves after 24 h. On the other hand, IC50 of the nanosilver on HADSCs, A-431, HaCaT, HEK-293, HeLa, and THP-1 was as low as 13.22, 5.96, 6.289, 12.74, 6.0, 5.6 ppm, respectively. Lastly, through intravenous administration of particles in female BalB/ mice and image analysis, we were able to get an overview of particle safety on mouse organs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


