有壳微生物取食生态模式的性状预测因子
IF 1.6
2区 生物学
Q4 MICROBIOLOGY
引用次数: 0
摘要
功能性状为生态策略和进化多样化提供了关键的见解。在这项研究中,我们分析了一个综合的性状数据集,以研究来自北全北极地区的雄性变形虫的摄食生态的形态学预测因子,重点研究了372种变形虫的变异。我们还研究了性状多样性是否反映了科水平上的分类丰富度。形态学特征包括壳长、壳宽、孔径尺寸、形状和覆盖。主成分分析(PCA)表明,轴1主要代表壳和孔径大小的变化,轴2主要代表整体形状比例的差异。细菌食性物种表现出最大的形态和分类多样性,跨越21科48属,混合营养型和捕食者占据了其更广泛形态空间的巢状亚群。回归分析表明,物种丰富度与保护特征(包括孔缘形态和棘的存在)的变化之间存在显著的相关性。决策树模型发现,虽然混合营养型和捕食型动物的分类准确率较低,但孔径宽长比是捕食策略的关键预测因子。未来的研究应将壳形态与系统发育数据结合起来,以加强对遗存变形虫的生态策略预测,并探索在更广泛的地理范围和不同环境下功能多样化的假设。本文章由计算机程序翻译,如有差异,请以英文原文为准。
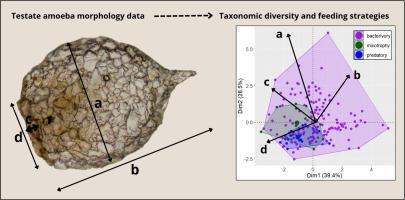
Trait-based predictors of feeding ecology patterns in shelled microorganisms
Functional traits provide key insights into ecological strategies and evolutionary diversification. In this study, we analyzed a comprehensive trait dataset to investigate morphological predictors of feeding ecology in testate amoebae from the Northern Holarctic realm, focusing on variability across 372 species. We also examined whether trait diversity mirrors taxonomic richness at the family level. Morphological traits included shell length, shell width, aperture dimensions, shape, and covering. The Principal Component Analysis (PCA) indicated that Axis 1 predominantly represented variation in shell and aperture size, while Axis 2 was associated with differences in overall shape proportions. Bacterivorous species exhibited the greatest morphological and taxonomic diversity, spanning 21 families and 48 genera, with mixotrophs and predators occupying nested subsets of their broader morphospace. The regression analyses demonstrated significant associations between species richness and variation in protective features, including aperture rim morphology and the presence of spines. Decision tree models identified the aperture width-to-length ratio as a key predictor of feeding strategy, although classification accuracy was lower for mixotrophs and predators. Future research should integrate shell morphology with phylogenetic data to enhance ecological strategy predictions in testate amoebae and explore hypotheses regarding functional diversification across a broader geographical scale and within different environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European journal of protistology
生物-微生物学
CiteScore
4.60
自引率
20.70%
发文量
55
审稿时长
14.6 weeks
期刊介绍:
Articles deal with protists, unicellular organisms encountered free-living in various habitats or as parasites or used in basic research or applications. The European Journal of Protistology covers topics such as the structure and systematics of protists, their development, ecology, molecular biology and physiology. Beside publishing original articles the journal offers a forum for announcing scientific meetings. Reviews of recently published books are included as well. With its diversity of topics, the European Journal of Protistology is an essential source of information for every active protistologist and for biologists of various fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


