ZIC-HILIC富集-高分辨率质谱(HRMS)技术鉴定灵芝N-和o -糖肽
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本研究对灵芝多糖肽(GL-PPSQ2)蛋白糖基化进行了综合鉴定。通过ZIC-HILIC富集,在nanoRPLC-ESI-MS/MS上通过高能碰撞解离(HCD)裂解分析完整的N-和o -糖肽。共鉴定出48个蛋白和62个多肽,其中57个是独特的肽段。使用pGlyco进行糖蛋白组学分析,从32种n -糖蛋白的35个n -糖位点中发现了42个完整的n -糖肽,而GPSeeker则鉴定了4个具有规则n -糖蛋白核心结构的完整n -糖肽。对于o -糖基化,pGlyco从160个o -糖蛋白的287个o -糖位点中注释了338个完整的o -糖肽,揭示了o -糖基化的显著优势和异质性。这些糖肽表现出不同的聚糖组成,包括高甘露糖、复杂和杂交类型,以及宏观和微观的异质性。本研究提出了GL-PPSQ2糖基化的直接质谱分析方法,增强了对糖基化修饰和糖蛋白研究的认识。研究结果阐明了GL-PPSQ2的糖基化模式和结构特征,为了解其生物学效应和开发利用灵芝多糖肽组分的功能食品提供了科学依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
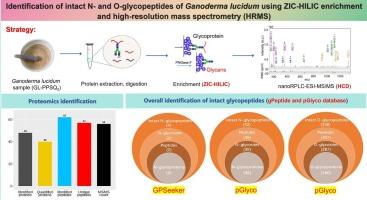
Identification of intact N- and O-glycopeptides of Ganoderma lucidum using ZIC-HILIC enrichment and high-resolution mass spectrometry (HRMS)
In this study, a comprehensive identification of protein glycosylation of G. lucidum polysaccharide peptide (GL-PPSQ2) was investigated. With ZIC-HILIC enrichment, intact N- and O-glycopeptides were analyzed by higher-energy collisional dissociation (HCD) fragmentation on nanoRPLC-ESI-MS/MS. A total of 48 proteins and 62 peptides were identified, including 57 unique peptide segments. Glycoproteomic profiling using pGlyco revealed 42 intact N-glycopeptides from 35 N-glycosites of 32 N-glycoproteins, whereas GPSeeker identified four intact N-glycopeptides with regular N-glycan core structures. For O-glycosylation, pGlyco annotated 338 intact O-glycopeptides from 287 O-glycosites of 160 O-glycoproteins, revealing a marked predominance and heterogeneity of O-glycosylation. These glycopeptides exhibited diverse glycan compositions, including high mannose, complex, and hybrid types, as well as macro- and microheterogeneity. This study presents direct MS analysis of GL-PPSQ2 glycosylation, enhancing the understanding of glycosylation modifications and glycoprotein research. The findings elucidate the glycosylation patterns and structural characteristics of GL-PPSQ2, providing scientific evidence for understanding its biological effects and supporting the development of functional foods based on G. lucidum polysaccharide peptide components.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


