TNF-α-预处理通过靶向Nav1.6的miR-101b-3p增强间充质干细胞源性细胞外囊泡对神经性疼痛的镇痛作用
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
间充质干细胞衍生的细胞外囊泡(MSC-EV)已显示出缓解疼痛的希望,但其疗效有限。用肿瘤坏死因子-α (TNF-α)预处理MSC可增强其治疗潜力;然而,对镇痛的影响和潜在的机制尚不清楚。在此,我们研究了TNF-α-预处理MSC (T-EV)的EV在慢性收缩损伤(CCI)小鼠模型中的镇痛作用,并探讨了其分子机制。鞘内注射后,T-EV比对照MSC-EV (C-EV)产生了更大的机械和热痛阈改善,在两周内实现了增强的疼痛缓解。全细胞膜片钳记录显示,T-EV显著降低了背根神经节(DRG)神经元的放电速率和动作电位幅度。RNA测序显示T-EV在miR-101b-3p中富集。在T-EV中沉默miR-101b-3p可消除其增强的镇痛作用并逆转DRG的高兴奋性。此外,荧光素酶检测显示miR-101b-3p可直接结合Nav1.6的3'UTR,抑制其表达。工程化的msc衍生的纳米囊泡过表达miR-101b-3p,复制了T-EV观察到的疼痛缓解增加。这些研究结果表明,TNF-α预处理通过传递miR-101b-3p提高MSC-EV的镇痛效力,miR-101b-3p下调Nav1.6,降低DRG高兴奋性。该研究支持mir -101b-3p富集囊泡作为治疗神经性疼痛的新策略的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
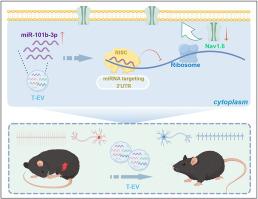
TNF-α-preconditioning enhances analgesic efficacy of mesenchymal stem cell-derived extracellular vesicle in neuropathic pain via miR-101b-3p targeting Nav1.6
Mesenchymal stem cell-derived extracellular vesicle (MSC-EV) has shown promise for pain relief, but its efficacy is limited. Preconditioning MSC with tumor necrosis factor-α (TNF-α) may enhance their therapeutic potential; however, the impact on analgesia and underlying mechanisms remains unclear. Here, we investigated the analgesic effects of EV from TNF-α-preconditioned MSC (T-EV) in a chronic constriction injury (CCI) mouse model and examined the molecular mechanisms involved. Following intrathecal injection, T-EV produced greater improvements in mechanical and thermal pain thresholds than control MSC-EV (C-EV), achieving enhanced pain relief for two weeks. Whole-cell patch-clamp recordings revealed that T-EV markedly decreased both the firing rate and action potential amplitude of dorsal root ganglion (DRG) neurons. RNA sequencing revealed that T-EV was enriched in miR-101b-3p. Silencing miR-101b-3p in T-EV abolished their enhanced analgesic effects and reversed DRG hyperexcitability. Moreover, miR-101b-3p was shown by luciferase assays to bind directly to the 3′UTR of Nav1.6, suppressing its expression. Engineered MSC-derived nanovesicle overexpressing miR-101b-3p replicated the increased pain relief observed with T-EV. These findings demonstrate that TNF-α preconditioning improves the analgesic potency of MSC-EV by delivering miR-101b-3p, which downregulates Nav1.6 and decreases DRG hyperexcitability. This study supports the therapeutic potential of miR-101b-3p-enriched vesicle as a novel strategy for treating neuropathic pain.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


