缺乏标准化的抗磷脂抗体检测可能有利于在抗磷脂综合征分类中使用第99百分位截止点
IF 3.4
3区 医学
Q2 HEMATOLOGY
Research and Practice in Thrombosis and Haemostasis
Pub Date : 2025-07-01
DOI:10.1016/j.rpth.2025.102967
引用次数: 0
摘要
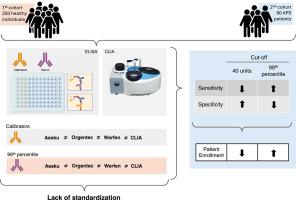
背景:美国风湿病学会/欧洲风湿病协会联盟发布的抗磷脂综合征(APS)分类标准要求3个分子靶点中的任何一个呈阳性:狼疮抗凝血剂、抗心磷脂(aCL)免疫球蛋白G或抗β2糖蛋白I (aβ 2gpi)免疫球蛋白G,后2个要求浓度>;40单位。该规范要求具有标准化和可比较的校准策略,以实现适当的患者分类。过去,校准器测试的标准化程度较差;因此,将第99个百分位作为分界点。目的:通过协调第99百分位和40单位阈值,在APS研究患者入组的实验室标准中找到敏感性和特异性之间的平衡。方法采用4种不同的方法(3种比色法、标准化ELISA平台和1种化学发光法)测定250名健康人群的aCL和a - β 2gpi浓度,确定第99百分位。我们测试了标准化校准器在试剂盒之间的交叉反应性,以及如何在80例APS患者队列中实现更好的患者入组准确性。结果我们发现,第99百分位基本上是40个单位的截止值,并且在确定的截止值中观察到相当大的试剂盒间差异,这源于试剂盒校准器的标准化不足。在第二组80例APS患者中,我们通过比较第99百分位和40个单位的截止值来估计这些不同方法的准确性。对于某些ELISA试剂盒,使用40个单位的固定截止值而不是第99百分位数会降低其敏感性,但不会增加特异性,这影响了患者的分类,从而影响了符合APS研究条件的患者数量。在第99百分位截止点用2个ELISA平台进行检测将提高患者的资格。结论在没有标准化的aCL或a - β 2gpi校准器的情况下,应采用2种不同ELISA试剂盒的第99百分位作为截止点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The absence of standardization in antiphospholipid antibody testing may favor the use of 99th percentile cutoffs in antiphospholipid syndrome classification
Background
Classification criteria for antiphospholipid syndrome (APS) issued by the American College of Rheumatology/European Alliance of Associations for Rhuematology necessitate a positivity for any of the 3 molecular targets: lupus anticoagulant, anticardiolipin (aCL) immunoglobulin G, or anti-β2 glycoprotein I (aβ2GPI) immunoglobulin G, with the latter 2 requiring concentrations > 40 units. This specification implies having standardized and comparable calibration strategies to achieve proper patient classification. In the past, calibrator tests suffered from poor standardization; thus, the 99th percentile was established as the cutoff point.
Objectives
We aimed to find a balance between sensitivity and specificity in the laboratory criteria for patient enrollment in APS studies by harmonizing the 99th percentile and 40-unit threshold.
Methods
In a cohort of 250 healthy individuals, we tested aCL and aβ2GPI concentrations by 4 different methods: 3 colorimetric, standardized ELISA platforms and 1 chemiluminescence assay, to define the 99th percentile. We tested cross-reactivity of standardized calibrators between kits and how to implement better accuracy for patient enrollment in a cohort of 80 APS patients.
Results
We found that the 99th percentile was substantially <40-unit cutoff and observed considerable interkit variability in the determined cutoffs, which originated from the inadequate standardization of kit calibrators. In a second cohort of 80 APS patients, we estimated the accuracy of these different methods by comparing the 99th percentile and 40-unit cutoffs. For certain ELISA kits, using a fixed cutoff of 40 units instead of the 99th percentile decreased their sensitivity without increasing specificity, which affected patient classification and thus the number of patients eligible for APS studies. Testing with 2 ELISA platforms at the 99th percentile cutoff would improve patient eligibility.
Conclusion
Our survey suggests that in the absence of standardized calibrators for testing aCL or aβ2GPI, a cutoff point at the 99th percentile of 2 different ELISA kits should be adopted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Research and Practice in Thrombosis and Haemostasis
Medicine-Hematology
CiteScore
5.60
自引率
13.00%
发文量
212
审稿时长
7 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


