磁性Fe3O4@Ti3AlC2复合材料对水中铀(VI)高效修复的协同吸附-还原机理
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-07-26
DOI:10.1016/j.jtice.2025.106316
引用次数: 0
摘要
背景铀(VI)具有高迁移性和高毒性,造成严重的环境和健康风险。现有的补救策略在效率和可扩展性方面面临挑战。本研究通过开发一种磁性Fe3O4@Ti3AlC2复合材料来解决这些限制,利用协同吸附-还原机制来有效去除U(VI)。方法采用共沉淀法将Fe3O4纳米颗粒整合到Ti3AlC2基体上,制备复合材料。间歇式吸附实验评估了不同pH、温度和共存离子条件下对U(VI)的去除效果。材料表征采用SEM, TEM, XRD, FTIR, XPS和BET分析来阐明结构和力学性能。拟二级动力学和Freundlich等温线模型证实,在pH为7时,吸附量最大为151.70 mg/g,主要由化学吸附和非均质表面的多层吸附驱动。机理分析表明,U(VI)通过氧化还原反应(部分还原为U(IV))和与表面官能团(如Ti-O, C = O)的络合而固定。该复合材料具有快速磁分离、可回收性和与天然地下水pH的相容性,无需严格的pH调节。值得注意的是,共存的Cu2+增强了吸附,而Pb2+和有机大分子抑制了吸附。这些发现表明Fe3O4@Ti3AlC2是一种可持续的、高效的铀修复吸附剂,在废水处理和环境恢复中提供了可扩展的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
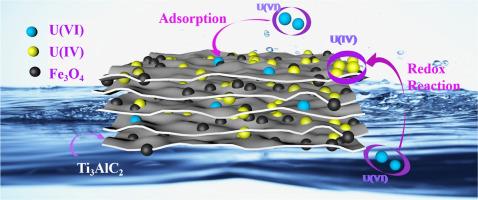
Synergistic adsorption-reduction mechanism of magnetic Fe3O4@Ti3AlC2 composites for high-efficiency uranium (VI) remediation in aqueous systems
Background
Uranium(VI) contamination poses severe environmental and health risks due to its high mobility and toxicity. Existing remediation strategies face challenges in efficiency and scalability. This study addresses these limitations by developing a magnetic Fe3O4@Ti3AlC2 composite, leveraging synergistic adsorption-reduction mechanisms for effective U(VI) removal.
Methods
The composite was synthesized via co-precipitation, integrating Fe3O4 nanoparticles onto Ti3AlC2 substrates. Batch adsorption experiments evaluated U(VI) removal efficiency under varied pH, temperature, and coexisting ion conditions. Material characterization employed SEM, TEM, XRD, FTIR, XPS, and BET analysis to elucidate structural and mechanistic properties.
Significant Findings
Optimal adsorption occurred at pH 7 with a maximum capacity of 151.70 mg/g, driven by chemisorption and multilayer adsorption on heterogeneous surfaces, as confirmed by pseudo-second-order kinetics and Freundlich isotherm models. Mechanistic analyses revealed U(VI) immobilization through redox reactions (partial reduction to U(IV)) and complexation with surface functional groups (e.g., Ti–O, C = O). The composite demonstrated rapid magnetic separation, recyclability, and compatibility with natural groundwater pH, eliminating the need for rigorous pH adjustment. Notably, coexisting Cu2+ enhanced adsorption, while Pb2+ and organic macromolecules inhibited performance. These findings establish Fe3O4@Ti3AlC2 as a sustainable, high-efficiency adsorbent for uranium remediation, offering scalable applications in wastewater treatment and environmental restoration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


