Maresin1通过抑制TLR4/MyD88/NF-kB通路改善胫骨骨折小鼠术后谵妄样行为
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
背景术后谵妄(POD)是老年患者手术后常见的并发症,导致发病率和医疗费用增加。虽然POD的病理生理机制尚不完全清楚,但它被认为与显著的炎症反应有关,特别是星形胶质细胞的激活。本研究探讨了Maresin1 (MaR1)单独或与TLR4抑制剂TAK-242合用对POD小鼠模型的神经保护作用。我们的研究结果表明,MaR1通过抑制TLR4/MyD88/NF-κB信号通路来减少星形胶质细胞的激活,从而减轻POD相关的炎症反应。这些结果表明,靶向星形胶质细胞TLR4/MyD88/NF-κB活化可能是一种有希望的预防和治疗POD的治疗策略。目的本研究旨在评估Maresin1(一种来源于omega-3脂肪酸DHA的生物活性分子)单独或与合成TLR4抑制剂TAK-242联合使用对胫骨骨折内固定所致POD小鼠模型的认知结局和炎症反应的影响。方法将90只C57BL/6小鼠进行胫骨骨折手术治疗,分为假手术、单纯手术、生理盐水手术、MaR1手术、TAK-242手术、MaR1和TAK-242联合手术6组。采用埋藏食物测试、Y迷宫测试和开阔场地测试评估认知功能。采用Western blotting、qRT-PCR、免疫荧光和透射电镜检测星形细胞活化和TLR4/MyD88/NF-κB通路的完整性。结果与假手术组相比,经MaR1预处理的小鼠术后谵妄样行为明显改善。此外,与单独使用TAK-242的阳性对照组相比,MaR1和TAK-242联合治疗改善了小鼠的谵妄样行为,并有效降低了小鼠大脑中星形胶质细胞的激活以及相关标记物(GFAP和S100β)的表达。此外,这些治疗可以调节TLR4/MyD88/NF-κB信号通路,这是一种潜在的神经保护机制,可以减轻手术创伤的影响,预防术后谵妄。结论:在POD小鼠模型中,无论是单独使用还是与TAK-242联合使用,MaR1都具有显著的抗炎和神经保护作用,可能是通过抑制TLR4/MyD88/NF-kB的激活来实现的。这些发现表明,靶向TLR4/MyD88/NF-kB炎症通路可能有助于预防或减轻手术患者的POD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
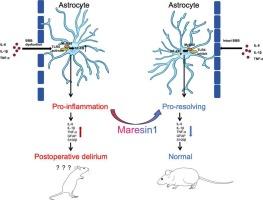
Maresin1 improves postoperative delirium-like behavior in mice with tibial fractures by inhibiting the TLR4/MyD88/NF-kB pathway
Background
Postoperative delirium (POD) is a common complication in elderly patients following surgery, contributing to increased morbidity and healthcare costs. Although the pathophysiological mechanisms of POD are not fully understood, it is believed to involve significant inflammatory responses, particularly the activation of astrocytes. This study investigates the neuroprotective potential of Maresin1 (MaR1), alone and in combination with the TLR4 inhibitor TAK-242, in a mouse model of POD. Our findings indicate that MaR1 reduces astrocyte activation by inhibiting the TLR4/MyD88/NF-κB signaling pathway, which mitigates inflammatory responses associated with POD. These results suggest that targeting astrocytic TLR4/MyD88/NF-κB activation could be a promising therapeutic strategy for POD prevention and treatment.
Purpose
This study aims to evaluate the effects of Maresin1 (MaR1), a bioactive molecule derived from the omega-3 fatty acid DHA, alone or in combination with TAK-242, a synthetic TLR4 inhibitor, on cognitive outcomes and inflammatory responses in a mouse model of POD induced by tibial fracture internal fixation.
Methods
Ninety C57BL/6 mice were subjected to tibial fracture surgery and divided into six groups to receive different treatments: sham, surgery only, surgery with normal saline, surgery with MaR1, surgery with TAK-242, and surgery with both MaR1 and TAK-242. Cognitive functions were assessed using the Buried Food Test, Y Maze Test and Open Field Test. Western blotting, qRT-PCR, immunofluorescence, and transmission electron microscopy were utilized to examine astrocytic activation and the integrity of the TLR4/MyD88/NF-κB pathway.
Results
Compared to the sham-operated group, mice pretreated with MaR1 exhibited significant improvements in postoperative delirium-like behavior. Furthermore, in contrast to the positive control group treated with TAK-242 alone, Combination treatment with MaR1 and TAK-242 improved the delirium-like behavior in mice, and effectively reduced the activation of astrocytes as well as the expression of associated markers (GFAP and S100β) in the mouse brain. Additionally, these treatments modulated the TLR4/MyD88/NF-κB signaling pathway, which serves as a potential neuroprotective mechanism to mitigate the impact of surgical trauma and prevent postoperative delirium.
Conclusion: MaR1, whether used alone or in combination with TAK-242, demonstrates significant anti-inflammatory and neuroprotective effects in a POD mouse model, achieved likely through the inhibition of TLR4/MyD88/NF-kB activation. These findings suggest that targeting TLR4/MyD88/NF-kB inflammatory pathway may help prevent or mitigate POD in surgical patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


