吡虫啉暴露下周围植物生物膜中多界微生物相互作用介导的氮损失的亚转录组学见解
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
水稻生态系统中,周围植物生物膜普遍存在,并驱动着生物地球化学养分循环。然而,多界微生物相互作用调节生物膜中氮循环的机制仍未被探索。本研究通过微观实验研究了新烟碱类农药吡虫啉(IMI)对周围植物生物膜N循环的影响。在30天的试验中,水-生物膜微生物系统损失了0.02 ~ 0.12 mg氮,随着IMI暴露浓度的增加而增加。亚转录组测序显示生物膜中硝化基因下调84.3% ~ 92.2%,反硝化基因上调33.9% ~ 143%。这些基因表达的改变分别受到与生物膜潜在硝化和反硝化活性相关的生态集群内微生物竞争和合作的调控。这些集群中微生物的相对丰度与基因丰度之间的相关性揭示了硝化和反硝化的多界群落,包括硝化作用的亚硝化菌(古细菌)和硝化螺旋菌(细菌),以及反硝化作用的肠球菌(细菌)和曲霉(真菌)。偏最小二乘路径模型表明,生物膜介导的氮损失是由促进反硝化作用(总效应= -0.83)而不是抑制硝化作用(总效应= 0.52)驱动的。我们的研究结果强调了多领域微生物相互作用调节周围植物生物膜的氮循环和新烟碱类农药的风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
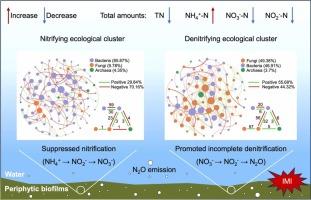
Metatranscriptomic insights into nitrogen loss mediated by multi-kingdom microbial interactions in periphytic biofilms under imidacloprid exposure
Periphytic biofilms are ubiquitous and drive biogeochemical nutrient cycling in paddy ecosystems. However, the mechanisms of multi-kingdom microbial interactions regulating nitrogen (N) cycling in the biofilms remain unexplored. This study conducted a microcosm experiment to investigate the effects of imidacloprid (IMI), a widely used neonicotinoid pesticide, on N cycling in periphytic biofilms. Throughout the 30-day experiment, 0.02 to 0.12 mg of N was lost from the water-biofilm microcosm systems, increasing with higher IMI exposure concentrations. Metatranscriptomic sequencing demonstrated 84.3%–92.2% downregulation of nitrification genes and 33.9%–143% upregulation of denitrification genes in the biofilms. These alterations in gene expression were regulated by microbial competition and cooperation within ecological clusters associated with the biofilm potential nitrification and denitrification activities, respectively. Correlation between the relative abundance of microorganisms in these clusters and gene abundance revealed multi-kingdom nitrifying and denitrifying communities, including Nitrosopumilus (Archaea) and Nitrospira (Bacteria) for nitrification, and Enterococcus (Bacteria) and Aspergillus (Fungi) for denitrification. Partial least squares path modeling indicated that the biofilm-mediated N loss was driven by promoted denitrification (total effect = -0.83) rather than suppressed nitrification (total effect = 0.52). Our findings highlight multi-kingdom microbial interactions regulating N cycling in periphytic biofilms and neonicotinoid pesticide risks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


