蝎毒耐热合成肽调节Nrf-2减轻PM2.5暴露下内质网应激诱导的AD模型神经元焦亡
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
近年来,细颗粒物(PM2.5)对神经系统的影响,特别是对神经退行性疾病的影响引起了广泛关注。流行病学研究表明,暴露于PM2.5与阿尔茨海默病(AD)的发生和发展密切相关。然而,PM2.5加剧AD的机制尚不清楚。蝎毒耐热合成肽(SVHRSP)是利用蝎毒耐热肽的氨基酸序列合成的具有药理活性的产品。在本研究中,我们研究了PM2.5暴露加重神经元焦亡的机制,并验证了SVHRSP对AD的保护作用。采用PC12和HT22神经元细胞构建AD体外模型。小鼠立体定向注射Aβ25-35蛋白诱导AD。随后,通过体内和体外实验检测PM2.5暴露引起的AD模型内质网应激、焦亡和氧化应激的变化。结果表明,PM2.5暴露加重了AD小鼠的认知障碍、内质网应激和焦亡。相反,用SVHRSP处理可以抵消PM2.5暴露引起的损伤。在机制层面上,PM2.5可能通过抑制Nrf-2的表达而增强氧化应激并引发焦亡。这些发现为PM2.5暴露加速阿尔茨海默病进展的机制提供了新的见解,并为阿尔茨海默病的药物治疗提供了有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
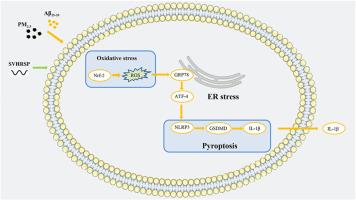
Scorpion venom heat-resistant synthetic peptide regulates Nrf-2 to alleviate neuronal pyroptosis in an AD model induced by endoplasmic reticulum stress under PM2.5 exposure
In recent years, the impact of fine particulate matter (PM2.5) on the nervous system, particularly on neurodegenerative diseases, has garnered widespread attention. Epidemiological studies have shown that exposure to PM2.5 is closely associated with the development and progression of Alzheimer's disease (AD). However, the mechanisms through which PM2.5 exacerbates AD remain unclear. Scorpion venom heat-resistant synthetic peptide (SVHRSP) is a pharmacologically active product synthesised from the amino acid sequence of scorpion venom heat-resistant peptide. In this study, we investigated the mechanism through which PM2.5 exposure aggravates neuronal pyroptosis and validated the protective effects of SVHRSP against AD. PC12 and HT22 neuronal cells were used to construct in vitro models of AD. Mice were stereotactically injected with Aβ25-35 protein to induce AD. Subsequently, PM2.5 exposure-induced changes in ER stress, pyroptosis, and oxidative stress in AD models, were detected through in vivo and in vitro experiments. The results indicated that PM2.5 exposure aggravated cognitive impairment, ER stress, and pyroptosis in mice with AD. Conversely, treatment with SVHRSP counteracted the damage induced by PM2.5 exposure. At a mechanistic level, PM2.5 might enhance oxidative stress and trigger pyroptosis by suppressing the expression of Nrf-2. These findings offer novel insights into the mechanisms through which PM2.5 exposure hastens the progression of AD and propose a promising strategy for the pharmacological treatment of AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


