MDM2和β-catenin同时抑制p53野生型肺腺癌的双靶向N-PMIplus@CA纳米平台
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
携带野生型p53的肺腺癌(LUAD)在治疗上仍然具有挑战性,因为致癌的mdm2驱动的p53活性抑制和Wnt/β-catenin信号的代偿激活。在这里,我们报告了一个自组装的纳米颗粒系统(N-PMIplus@CA),可以同时抑制MDM2和Wnt/β-catenin途径。我们观察到,这两种通路的同时激活与LUAD患者的不良临床结果显著相关。N-PMIplus@CA通过增强渗透性和滞留性(EPR)效应和巨噬细胞作用在肿瘤组织中有效积累,提供优化的mdm2抑制肽和β-catenin拮抗剂(鼠尾草酸)。双抑制策略在LUAD细胞系和小鼠模型中显著恢复p53功能,下调β-catenin信号,抑制肿瘤增殖,促进细胞凋亡。值得注意的是,与单药治疗对照组相比,N-PMIplus@CA治疗在皮下和原位LUAD模型中具有更好的抗肿瘤疗效,没有可检测到的全身毒性。通过共包实现的统一的药代动力学特征确保了同步的细胞内递送和肿瘤部位的最大协同效应。这种纳米医学方法有效地规避了传统双药治疗的局限性,如代谢差异和全身不良反应。总之,我们的研究结果突出了N-PMIplus@CA作为一个有希望的临床候选者,解决了p53野生型LUAD治疗中关键的未满足需求,展示了强大的翻译潜力,并提供了同时靶向多种致癌途径的范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
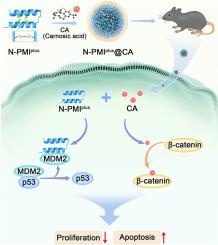
Dual-targeted N-PMIplus@CA nanoplatform for concurrent MDM2 and β-catenin inhibition in p53 wild-type lung adenocarcinoma
Lung adenocarcinoma (LUAD) harboring wild-type p53 remains therapeutically challenging because of the oncogenic MDM2-driven suppression of p53 activity and compensatory activation of Wnt/β-catenin signaling. Here, we report a self-assembled nanoparticle system (N-PMIplus@CA) enabling simultaneous inhibition of both MDM2 and the Wnt/β-catenin pathway. We observed that concurrent activation of these two pathways is significantly correlated with poor clinical outcomes in patients with LUAD. N-PMIplus@CA efficiently accumulated in tumor tissues via enhanced permeability and retention (EPR) effects and macropinocytosis, delivering both an optimized MDM2-inhibitory peptide and a β-catenin antagonist (carnosic acid). The dual-inhibition strategy significantly restored p53 function, downregulated β-catenin signaling, suppressed tumor proliferation, and promoted apoptosis in LUAD cell lines and murine models. Remarkably, treatment with N-PMIplus@CA yielded superior antitumor efficacy in subcutaneous and orthotopic LUAD models compared with monotherapy controls, without detectable systemic toxicity. The unified pharmacokinetic profile achieved by coencapsulation ensured synchronized intracellular delivery and maximal synergistic effects at tumor sites. This nanomedicine approach effectively circumvents traditional dual-drug therapy limitations, such as differential metabolism and systemic adverse effects. Collectively, our findings highlight N-PMIplus@CA as a promising clinical candidate that addresses a critical unmet need in p53 wild-type LUAD treatment, demonstrating strong translational potential and providing a paradigm for simultaneous targeting of multiple oncogenic pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


