锡有机骨架催化外映异构合成稀有糖的研究
Q2 Materials Science
Current Research in Green and Sustainable Chemistry
Pub Date : 2025-01-01
DOI:10.1016/j.crgsc.2025.100476
引用次数: 0
摘要
易获得的单糖的外聚化提供了一种原子高效的方法来扩大稀有单糖的组合。在这里,我们报道了锡有机框架(Sn-OF)作为一种高选择性催化剂,通过外映异构反应合成稀有单糖,如l -核糖、d -葡萄糖、D-talose和L-quinovose。外聚化产物的选择性达到67 ~ 95%。得到的最大产率为:d -葡萄糖22%,l -核糖14%,D-talose 15%, l -藜麦糖18%。此外,底物的结构与反应速率之间建立了相关性,表明糖以开链形式反应,羟基的顺式取向有利于外映化。结果表明,该催化剂可在二次循环中重复使用。催化外映反应产生外映体混合物,通过结晶可以实现底物的部分回收,如l -阿拉伯糖、d -半乳糖和d -木糖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
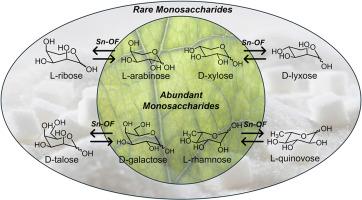
Synthesis of rare sugars via epimerization catalyzed by tin-organic frameworks
Epimerization of readily available monosaccharides presents an atom-efficient approach to expand the portfolio of rare monosaccharides. Here, we report tin-organic frameworks (Sn-OF) as a highly selective catalyst for synthesis of rare monosaccharides, such as L-ribose, D-lyxose, D-talose, and L-quinovose, via epimerization reaction. Remarkable selectivity of 67–95 % for the epimerization products was achieved. The maximal yields obtained were 22 % for D-lyxose, 14 % for L-ribose, 15 % for D-talose, and 18 % for L-quinovose. Additionally, a correlation between the structure of the substrate and reaction rate was established, suggesting that the saccharides react in open-chain form, with the cis-orientation of OH-groups facilitating the epimerization. Moreover, it was shown that the catalyst can be reused in a second run. The catalytic epimerization results in a mixture of epimers, from which partial recovery of the substrate can be achieved through crystallization, as demonstrated for L-arabinose, D-galactose, and D-xylose.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Green and Sustainable Chemistry
Materials Science-Materials Chemistry
CiteScore
11.20
自引率
0.00%
发文量
116
审稿时长
78 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


