金催化烯烃氧芳化制二氢呋喃融合萘醌的途径
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
提出了一种配体辅助Au(I)/Au(III)催化合成二氢呋喃融合萘醌的方法,以实现烯烃的1,2-氧芳化反应。这种氧化还原-中性方法利用了MeDalPhosAuCl的π酸性质,在温和的条件下促进1,2-双官能化,不需要外部氧化剂或光催化剂。该策略具有广泛的底物范围,采用易于获得的非预官能化芳基碘化物,并为结构复杂的杂环提供了有价值的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
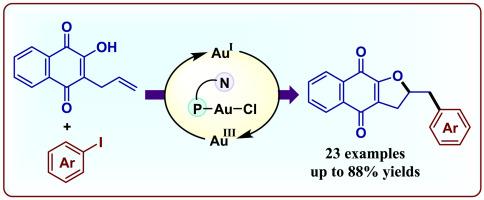
A route to dihydrofuran fused naphthoquinones via gold-catalyzed oxyarylation of alkenes
A ligand-assisted Au(I)/Au(III) catalytic approach has been developed to achieve 1,2-oxyarylation of alkenes for the efficient synthesis of dihydrofuran-fused naphthoquinones. This redox-neutral method leverages the π-acidic properties of MeDalPhosAuCl to facilitate 1,2-difunctionalization under mild conditions, eliminating the need for external oxidants or photocatalysts. The strategy exhibits broad substrate scope, employing readily available, non-prefunctionalized aryl iodides, and provides a valuable route to structurally complex heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


