口腔粘膜下纤维化的黏附药物输送系统在三维多细胞和体内模型:新方法的翻译治疗
IF 2.9
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
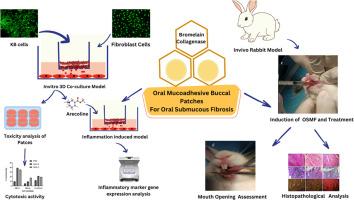
目的:本研究旨在开发和评估基于蛋白酶的黏附口腔贴片- B贴片(单独菠萝蛋白酶)和C贴片(菠萝蛋白酶与胶原酶)-用于口腔粘膜下纤维化(OSMF)的无创治疗。研究设计分析贴片的理化性质、酶活性及治疗效果。在模拟OSMF微环境的KB-3-1口腔癌细胞和McCoy成纤维细胞三维共培养模型中,槟油碱诱导纤维化和炎症。采用osmf诱导的家兔模型评估临床结果。结果B贴片(pH 6.8 ~ 7.03)和C贴片(pH 5.92 ~ 6.98)水化迅速,溶胀平衡;由于协同酶活性,Patch C表现出增强的胶原降解,而Patch B具有更好的生物相容性(溶血率0.57%),并在6个月后保持菠萝蛋白酶活性。在3D共培养模型中,两种贴片均显著下调槟槟碱诱导的MMP-2、MMP-9、IL-6和IV型胶原蛋白的表达,显示出其抗炎和抗纤维化的潜力。在体内,组织病理学分析证实,贴片改善了口腔张开,降低了纤维化的严重程度,胶原沉积明显减少。Patch B显示出对癌细胞的选择性细胞毒性,对正常成纤维细胞的影响最小。结论基于菠萝蛋白酶的口腔贴片通过减少纤维化和炎症,同时最小化全身效应,为OSMF提供了一种靶向性、非侵入性的治疗方法。由于它们在临床前动物中的有效性和安全性,这些贴片为未来的人类临床试验和OSMF的非手术治疗提供了重要的转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mucoadhesive drug delivery system for oral submucous fibrosis in three-dimensional multicellular and in vivo models: novel approach towards translational therapeutics
Objective
This study aimed to develop and evaluate protease enzyme-based mucoadhesive buccal patches—Patch B (bromelain alone) and Patch C (bromelain with collagenase)—for the non-invasive management of Oral Submucous Fibrosis (OSMF).
Study design
The physicochemical properties, enzymatic activity, and therapeutic efficacy of the patches were analyzed. Arecoline was used to induce fibrosis and inflammation in a 3D co-culture model of KB-3–1 oral cancer cells and McCoy fibroblasts, mimicking the OSMF microenvironment. An OSMF-induced rabbit model was used to assess clinical outcomes.
Results
Patch B (pH 6.8–7.03) and Patch C (pH 5.92–6.98) exhibited rapid hydration and equilibrium swelling. Patch C showed enhanced collagen degradation due to synergistic enzymatic activity, while Patch B had superior biocompatibility (0.57 % haemolysis) and retained bromelain activity after six months. In the 3D co-culture model, arecoline-induced upregulation of MMP-2, MMP-9, IL-6, and collagen type IV was significantly downregulated by both patches, demonstrating their anti-inflammatory and anti-fibrotic potential. In vivo, the patches improved mouth opening and reduced the severity of fibrosis, as confirmed by histopathological analysis, which showed significant reductions in collagen deposition. Patch B demonstrated selective cytotoxicity toward cancer cells, with minimal impact on normal fibroblasts.
Conclusion
Bromelain-based buccal patches provide a targeted, non-invasive therapeutic approach for OSMF by reducing fibrosis and inflammation while minimizing systemic effects. Due to their effectiveness and safety profile in preclinical animals, these patches provide significant translational potential for future human clinical trials and non-surgical therapy of OSMF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materialia
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
6.40
自引率
2.90%
发文量
345
审稿时长
36 days
期刊介绍:
Materialia is a multidisciplinary journal of materials science and engineering that publishes original peer-reviewed research articles. Articles in Materialia advance the understanding of the relationship between processing, structure, property, and function of materials.
Materialia publishes full-length research articles, review articles, and letters (short communications). In addition to receiving direct submissions, Materialia also accepts transfers from Acta Materialia, Inc. partner journals. Materialia offers authors the choice to publish on an open access model (with author fee), or on a subscription model (with no author fee).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


