一个结构开关揭示了如何解除USP30并解锁有丝分裂
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
最近,一种高分辨率的USP30与选择性抑制剂结合的结构确定了一个通过酶的催化结构域的构象开关形成的隐结合袋。这种机制的洞察力为结构导向的有丝分裂增强化合物的设计打开了一扇门。本文章由计算机程序翻译,如有差异,请以英文原文为准。
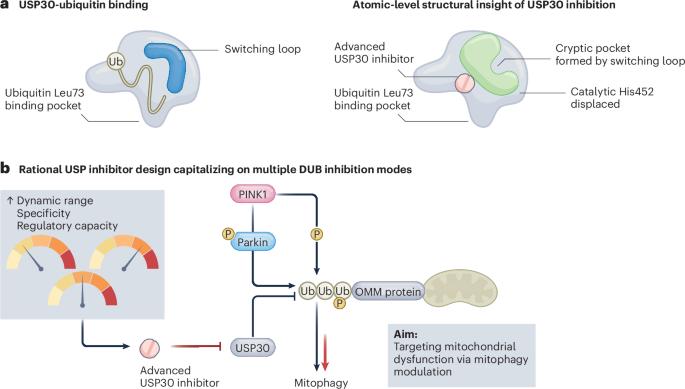

A structural switch reveals how to disarm USP30 and unlock mitophagy
A recent high-resolution structure of USP30 bound to a selective inhibitor identifies a cryptic binding pocket formed through a conformational switch in the catalytic domain of the enzyme. This mechanistic insight opens a door to structure-guided design of mitophagy-enhancing compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


