二甲基磺丙酸裂解酶基因dddY的克隆、表达及其活性所需关键氨基酸的鉴定
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
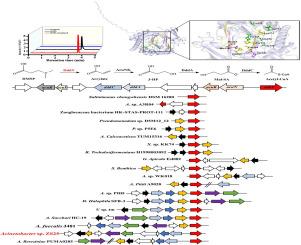
丰富的二甲基磺丙酸(DMSP)溶质在海洋生态系统中起着至关重要的作用。本研究分离到了一种能够完全矿化DMSP并产生DMS和丙烯酸酯的不动杆菌sp. ZS25。初步确定了DMSP可能的降解途径。通过基因组比较、基因敲除和异源表达相结合的方法确定DMSP裂解酶DddY的作用。DMSP对AsDddY的Km和kcat分别为2.6 mM和12.7 × 103 s−1。利用定点诱变技术检测AsDddY中特定氨基酸残基(Thr131、Asp181、Tyr225、Gly230、Gly250、His263、His265、Glu269、Tyr271、Leu274、Tyr331和His338)的影响,阐明它们在蛋白质功能中的关键作用。生物信息学分析发现19种不同的acdy - dddy聚类,而具有完整的dddY-acu聚类的菌株数量有限。我们的研究结果为DMSP生物降解的机制提供了新的见解,并增强了我们对天然细菌群体中存在的acu-dddY簇多样性的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cloning and expression of the dimethylsulfoniopropionate lyase gene dddY and the identification of the key amino acids necessary for its activity
The abundant solute dimethylsulfoniopropionate (DMSP) is crucial in marine ecosystems. In this study, a bacterium, Acinetobacter sp. ZS25, capable of completely mineralizing DMSP and producing DMS and acrylate, was isolated. The possible DMSP degradation pathway was primarily identified. The role of DMSP lyases DddY was identified through a combination of genomic comparison, gene knockout and heterologous expression. The Km and kcat of AsDddY for DMSP were 2.6 mM and 12.7 × 103 s−1, respectively. Site-directed mutagenesis was employed to examine the influence of specific amino acid residues (Thr131, Asp181, Tyr225, Gly230, Gly250, His263, His265, Glu269, Tyr271, Leu274, Tyr331, and His338) within AsDddY, elucidating their critical roles in the protein's functionality. Bioinformatics analysis revealed 19 distinct acu-dddY cluster order types, while the number of strains with a complete dddY-acu cluster is limited. Our findings offer novel insights into the mechanisms underlying DMSP biodegradation and enhance our understanding of the diversity of acu-dddY clusters present in natural bacterial populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


