利用外消旋和对映选择性磺胺酸拴链aza-Michael环化反应合成天然和非天然β-氨基酸
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们详细介绍了10个β-氨基酸(7个外消旋和3个标度)的文库的制备,重点介绍了我们实验室的外消旋和对映选择性氨基磺酸系扎嘧啶-迈克尔环化反应。所有十种合成都是由我们实验室发明的反应提供的恶噻嗪烷杂环进行的。这项工作提出了突出的磺胺酸系缚aza-Michael技术,并主张在烯烃功能化过程中更普遍地使用磺胺酸盐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
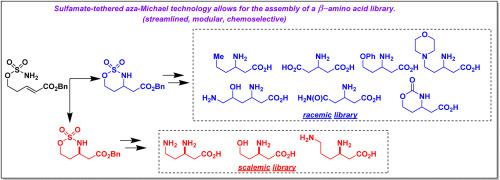
Syntheses of natural and non-natural β-amino acids using racemic and enantioselective sulfamate-tethered aza-Michael cyclization reactions
We detail the preparation of a library of ten β-amino acids (7 racemic and 3 scalemic), highlighting our laboratory's racemic and enantioselective sulfamate-tethered aza-Michael cyclization reactions. All ten syntheses proceed from oxathiazinane heterocycles furnished by reactions invented in our laboratory. This work raises the prominence of sulfamate-tethered aza-Michael technology and advocates for the more general use of sulfamates in olefin functionalization processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


