聚离子液体-丙烯酸-改性MIL-101(Cr)金属有机骨架:制备及对铕的高效吸附
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
以金属有机骨架、聚合物离子液体和丙烯酸为材料,合成了一种新型复合材料Poly(IL-AA)@MIL-101(Cr),用于选择性高效吸附稀土铕(III) (Eu3+)。利用x射线衍射(XRD)、傅里叶变换红外(FTIR)、扫描电镜-能量色散x射线能谱(SEM-EDS)、热重分析(TGA)和布鲁诺尔-埃米特-泰勒(BET)等技术对材料进行了表征。结果表明,聚合物离子液体在保留MIL-101(Cr)晶体结构和形貌的同时,成功地结合到材料表面。吸附实验探讨了平衡pH值、初始Eu3+浓度和持续时间等参数,并对吸附动力学、等温线和机理进行了综合分析。结果表明,Poly(IL1-AA)@MIL-101(Cr)、Poly(IL3-AA)@MIL-101(Cr)和Poly(IL5-AA)@MIL-101(Cr)对Eu3+的吸附在约9 h时达到平衡,平衡pH为6.2。对Eu3+的吸附主要遵循拟二级动力学模型和Langmuir等温吸附模型。此外,制备的复合材料对Eu3+的吸附选择性优于混合物中的其他金属离子(K+、Mg2+、Ni2+、Co2+、Zn2+、La3+和Nd3+)。即使经过5次吸附-解吸循环,复合材料仍能保持良好的吸附性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
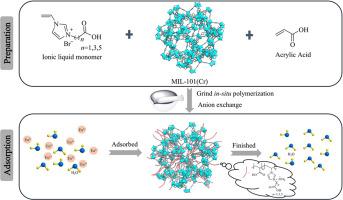
Poly(ionic liquids-acrylic acid)-modified MIL-101(Cr) metal−organic frameworks: Preparation and efficient adsorption of europium
A novel composite material, Poly(IL-AA)@MIL-101(Cr), combining metal–organic framework, polymeric ionic liquid and acrylic acid, was synthesized for the selective and efficient adsorption of rare earths europium(III) (Eu3+). Characterization of the materials was carried out using techniques such as X-ray diffraction (XRD), Fourier transform infrared (FTIR), scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS), thermogravimetric analysis (TGA) and Brunauer–Emmett–Teller (BET). The results demonstrate successful incorporation of the polymeric ionic liquid onto the material surface while preserving the crystal structure and morphology of MIL-101(Cr). Adsorption experiments were conducted to explore parameters including equilibrium pH, initial Eu3+ concentration, and duration, with comprehensive analyses of adsorption kinetics, isotherms, and mechanisms. Findings reveal that Poly(IL1-AA)@MIL-101(Cr), Poly(IL3-AA)@MIL-101(Cr), and Poly(IL5-AA)@MIL-101(Cr) achieve adsorption equilibrium for Eu3+ at approximately 9 h with an equilibrium pH of 6.2. The adsorption of Eu3+ predominantly follows a pseudo-second-order kinetic model and Langmuir isotherm adsorption model. Moreover, the prepared composite material exhibits superior adsorption selectivity for Eu3+ over other metal ions in the mixture (K+, Mg2+, Ni2+, Co2+, Zn2+, La3+, and Nd3+). Even after five adsorption–desorption cycles, the composite material maintains satisfactory adsorption performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


