影响铝颗粒燃烧特性关键因素的热力学和实验研究
IF 4.3
2区 材料科学
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
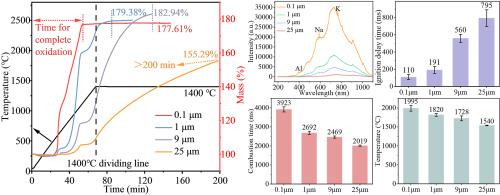
虽然铝颗粒在航空航天和导弹技术中得到了广泛的应用,但影响其燃烧的关键因素研究还不够。为了解决这一问题,本工作通过热力学理论计算和实验方法研究了多种因素对铝颗粒燃烧的影响。热力学结果表明,各种氧化性气体都能与铝发生化学反应并释放热量。其中,Al与氧的反应焓变和吉布斯自由能变化最大。在Al/O和Al/O/C/H体系中,温度升高降低了焓变,但提高了吉布斯自由能。压力对体系的影响可以忽略不计,而Al摩尔量的影响则取决于氧原子的可用性。热分析表明,较小的颗粒尺寸显著增加氧化速率。此外,不同粒径的Al颗粒在空气中1400℃的恒定温度下可以继续反应直至完全氧化,这表明高温下的氧化铝壳可能是疏松多孔的,结构不致密。燃烧试验表明,将颗粒尺寸从25 μm减小到0.1 μm,燃烧延迟时间减少86%,燃烧温度提高30%。同样,提高氧气浓度或压力可以减少点火延迟,提高燃烧温度,提高燃烧效率。该研究为构建复杂多变环境下的Al颗粒燃烧模型提供了基础数据支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermodynamic and experimental investigation of critical factors influencing the combustion characteristics of aluminum particles
Although aluminum (Al) particles have been widely applied in aerospace and missile technologies, the critical factors influencing their combustion remain insufficiently studied. To address this, this work investigates the effects of multiple factors on Al particle combustion through thermodynamic theoretical calculations and experimental approaches. Thermodynamic results indicate that various oxidizing gases can chemically react with Al and release heat. Among them, the reaction between Al and oxygen exhibits the largest enthalpy change and Gibbs free energy change. In both Al/O and Al/O/C/H systems, increasing temperature reduces enthalpy change but enhances Gibbs free energy change. Pressure shows negligible effects on the system, while the influence of Al molar quantity depends on oxygen atom availability. Thermal analysis reveals that smaller particle sizes significantly increase oxidation rates. Moreover, Al particles with different particle sizes can continue to react until complete oxidation at a constant temperature of 1400 °C in air, suggesting that the alumina shell at high temperatures may be loose and porous with a non-dense structure. Combustion tests demonstrate that reducing particle size from 25 μm to 0.1 μm decreases ignition delay time by 86 % and increases combustion temperature by 30 %. Similarly, elevating oxygen concentration or pressure reduces ignition delay, enhances combustion temperature, and improves combustion efficiency. This study provides fundamental data support for constructing Al particle combustion models under complex variable environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Particuology
工程技术-材料科学:综合
CiteScore
6.70
自引率
2.90%
发文量
1730
审稿时长
32 days
期刊介绍:
The word ‘particuology’ was coined to parallel the discipline for the science and technology of particles.
Particuology is an interdisciplinary journal that publishes frontier research articles and critical reviews on the discovery, formulation and engineering of particulate materials, processes and systems. It especially welcomes contributions utilising advanced theoretical, modelling and measurement methods to enable the discovery and creation of new particulate materials, and the manufacturing of functional particulate-based products, such as sensors.
Papers are handled by Thematic Editors who oversee contributions from specific subject fields. These fields are classified into: Particle Synthesis and Modification; Particle Characterization and Measurement; Granular Systems and Bulk Solids Technology; Fluidization and Particle-Fluid Systems; Aerosols; and Applications of Particle Technology.
Key topics concerning the creation and processing of particulates include:
-Modelling and simulation of particle formation, collective behaviour of particles and systems for particle production over a broad spectrum of length scales
-Mining of experimental data for particle synthesis and surface properties to facilitate the creation of new materials and processes
-Particle design and preparation including controlled response and sensing functionalities in formation, delivery systems and biological systems, etc.
-Experimental and computational methods for visualization and analysis of particulate system.
These topics are broadly relevant to the production of materials, pharmaceuticals and food, and to the conversion of energy resources to fuels and protection of the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


