甘氨酸的仿生醛醇反应
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
甘氨酸与醛的对映选择性醛醇反应——直接进入一类重要的非规范氨基酸——迄今为止还没有小分子催化作用。现在,模仿苏氨酸醛缩酶的辅助因子,一种手性羰基催化剂已经被开发出来,它对这个反应非常有效。这种不对称协议已成功地应用于一千多种醛基物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
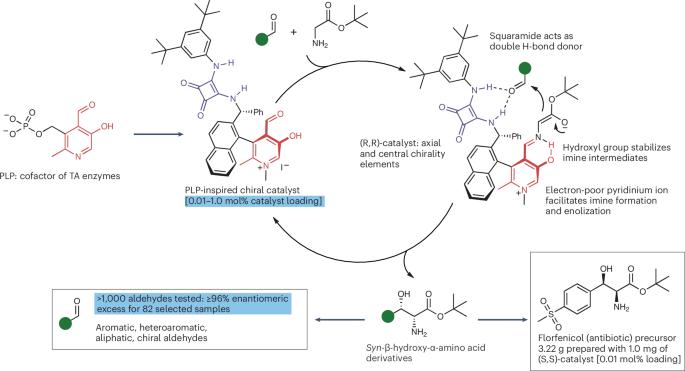
Biomimetic aldol reaction of glycinate
The enantioselective aldol reaction of glycinates with aldehydes — a direct entry to an important class of noncanonical amino acids — has so far eluded small-molecule catalysis. Now, mimicking the cofactor of threonine aldolase enzymes, a chiral carbonyl catalyst that is remarkably effective for this reaction has been developed. This asymmetric protocol has been successfully applied to more than a thousand aldehyde substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


