光氧化还原自由基休战-微笑重排n -亚砜酰丙烯酰胺与亚砜酰铵
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了可见光诱导n -亚砜酰丙烯酰胺与亚砜酰铵的休战-微笑重排反应。该反应在温和的条件下通过自由基加成/芳基迁移进行,以中高收率合成了一系列有价值的α-芳基酰胺。得到的α-芳基-γ-苯甲酰酰胺经过后期修饰转化为许多有合成价值的衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
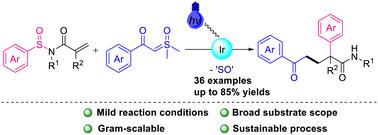
Photoredox radical Truce–Smiles rearrangement of N-sulfinyl acrylamides with sulfoxonium ylides†
The visible light-induced Truce–Smiles rearrangement reaction of N-sulfinyl acrylamides with sulfoxonium ylides is herein reported. The reaction is performed under mild conditions by radical addition/aryl migration, allowing the synthesis of a range of valuable α-arylamides in moderate to good yields. The resultant α-aryl-γ-benzoylamides were converted by late-stage modification to a number of synthetically valuable derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


