脑转移的代谢适应
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
脑转移瘤仍然是一个主要的临床挑战,其特点是高死亡率和通常有限的治疗选择。驱动脑转移的细胞和分子过程非常复杂,动态代谢适应使肿瘤细胞能够在大脑独特的微环境中茁壮成长。新出现的临床和临床前证据表明,这些代谢适应不是统一的,而是根据肿瘤起源组织、肿瘤基因组景观和承受营养压力的能力而变化。值得注意的是,大脑中的增殖和休眠转移细胞表现出不同的代谢特征,突出了靶向这些细胞的复杂性。关键的代谢途径,包括葡萄糖、脂肪酸和氨基酸代谢,不仅可以维持癌细胞的生存和生长,还可以调节与常驻脑细胞的相互作用,重塑其功能以支持转移。重要的是,这种代谢异质性强调了一刀切的治疗方法的不足。在这里,我们回顾了促进脑转移的适应性代谢重编程,并讨论了针对预防和治疗显性脑转移的量身定制干预措施的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
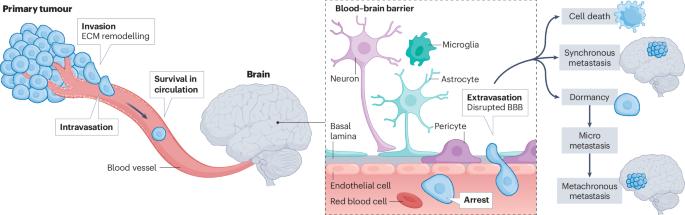
Metabolic adaptations of brain metastasis
Brain metastases remain a major clinical challenge, characterized by high mortality rates and often limited therapeutic options. The cellular and molecular processes that drive brain metastases are highly intricate, underscored by dynamic metabolic adaptations that enable tumour cells to thrive in the unique microenvironment of the brain. Emerging clinical and preclinical evidence reveals that these metabolic adaptations are not uniform but vary based on the tumour’s tissue of origin, oncogenomic landscape and capacity to endure nutrient stress. Notably, proliferative and dormant metastatic cells within the brain exhibit distinct metabolic profiles, highlighting the complexity of targeting these cells. Key metabolic pathways, including glucose, fatty acid and amino acid metabolism, are co-opted not only to sustain cancer cell survival and growth but also to modulate interactions with resident brain cells, reshaping their function to support metastasis. Importantly, this metabolic heterogeneity underscores the inadequacy of a one-size-fits-all therapeutic approach. Here, we review the adaptive metabolic reprogramming that facilitates brain metastases and discuss emerging strategies to tailor interventions aimed at preventing and treating overt brain metastases. In this Review, Parida and Malladi summarize the metabolic adaptations of tumour cells upon dissemination to the brain, outline the metabolic crosstalk between cancer and brain-resident cells and discuss potential strategies to target these adaptations to improve outcomes for patients with brain metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


