尺蠖的迁移有助于变形虫细胞适应高粘附环境
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
细胞动态调整其迁移模式以适应环境条件,但它们对粘性表面的反应仍不清楚,在粘性表面它们可能会被固定。在我们的研究中,我们发现强烈的粘附会促使盘状盘基骨柱发生实质性的变化,导致“尺蠖迁移”,这是变形虫迁移的一种新亚型。这种适应性包括细胞与表面之间的最小接触,细胞体直立并扭曲,然后快速重新附着以进行定向运动。同时,细胞脱落带有负电荷分子和粘附受体的迁移体,控制其粘附特性以恢复假足的迁移。我们确定了细胞质分裂机制的迁移模式转移和选择性膜脱落的重新利用是一个关键机制。中性粒细胞在强黏附下也表现出尺蠖迁移,提示其在变形虫细胞中广泛应用于适应高黏附环境。我们的发现阐明了变形虫细胞的一种程序化的适应性反应,可以有效地通过强粘性的地形。本文章由计算机程序翻译,如有差异,请以英文原文为准。
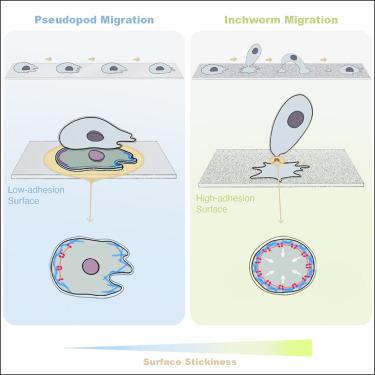
Inchworm migration facilitates amoeboid cell adaptation to high-adhesion environments
Cells dynamically adapt their migration modes to environmental conditions, but their response to sticky surfaces, where they risk becoming immobilized, remains unclear. In our study, we discovered that strong adhesion prompts substantial changes in Dictyostelium discoideum, leading to “inchworm migration,” a novel subtype of amoeboid migration. This adaptation involves minimal contact between the cell and the surface, with the cell body standing upright and twisting, followed by rapid reattachment for directed movement. Concurrently, the cells shed migrasomes loaded with negatively charged molecules and adhesion receptors, controlling their adhesion traits to resume pseudopod migration. We identify the repurposing of cytokinesis machinery for migration mode shifting and selective membrane shedding as a crucial mechanism. Neutrophils also exhibit inchworm migration under strong adhesion, suggesting its broader application among amoeboid cells in adapting to high-adhesion environments. Our findings illuminate a programmed, adaptive response in amoeboid cells to navigate effectively through strongly adhesive terrains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


