人脐带间充质干细胞外泌体在支气管肺发育不良中的保护机制。
摘要
支气管肺发育不良(BPD)的特征是肺泡形成减少和基质重塑紊乱。目前,尚无有效的治疗方法。本研究旨在探讨人脐带间充质干细胞外泌体通过调节巨噬细胞的免疫应答和炎症通路对BPD的保护作用。将pkh26标记的人脐带间充质干细胞系外体(hUCMSC-Exos)与RAW264.7细胞共培养,分为常氧组、常氧组+ NLRP3激活剂(尼日利亚蛋白)组、常氧组+ hUCMSC-Exos +尼日利亚蛋白组、高氧组、高氧组+ hUCMSC-Exos组、高氧组+ hUCMSC-Exos组和高氧组+ hUCMSC-Exos +尼日利亚蛋白组。测定各组细胞活力和细胞上清液中细胞因子的表达。PKH26外泌体染色证实RAW264.7细胞成功摄取hUCMSC-Exos。hUCMSC-Exos对尼日利亚菌素和高氧诱导的细胞活力降低具有保护作用。与常氧组相比,高氧组细胞中炎症因子IL-33、IL-6、il -1 β、tnf - α和IL-18的表达显著增加,NLRP3、ASC、caspase-1、IL-18、il -1 β和ATF4的mRNA和蛋白水平升高。与高氧组相比,高氧+ hUCMSC-Exos组IL-33、IL-6、il -1 β、tnf - α、IL-18和IL-33、IL-6、il -1 β、tnf - α和IL-18的表达降低。相比之下,与高氧组相比,高氧组+ hUCMSC-Exos + Nigericin组IL-33、IL-6、il -1 β、tnf - α和IL-18水平升高,NLRP3、ASC、caspase-1、IL-18、il -1 β和ATF4表达增加。hUCMSC-Exos通过降低内质网应激、抑制NLRP3炎性体表达和调节炎症细胞因子释放来减轻高氧诱导的肺巨噬细胞损伤,这可能对BPD有潜在的作用。支气管肺发育不良外泌体人脐带间充质干细胞巨噬细胞NLRP3
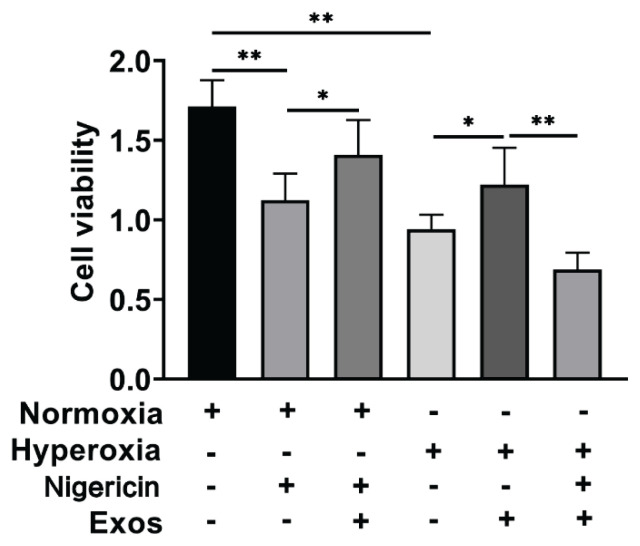
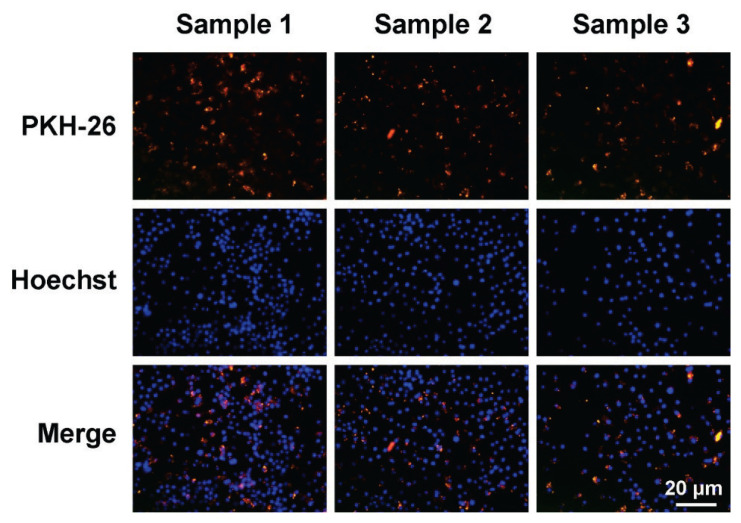
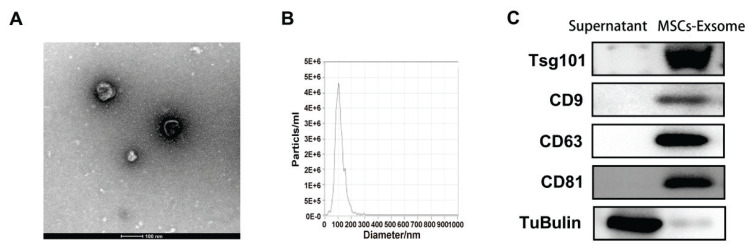
Bronchopulmonary dysplasia (BPD) is characterized by reduced alveolar formation and disordered matrix remodeling. Currently, there are no effective therapeutic approaches for it. This study aims to explore the protective effect of exosomes derived from human umbilical cord mesenchymal stem cells on BPD by regulating the immune response and inflammatory pathways of macrophages. PKH26-labeled human umbilical cord mesenchymal stem cell line exosomes (hUCMSC-Exos) were co-cultured with RAW264.7 cells, which were assigned to the following groups: normoxia, normoxia + NLRP3 activator (Nigericin), normoxia + hUCMSC-Exos + Nigericin, hyperoxia, hyperoxia + hUCMSC-Exos, and hyperoxia + hUCMSC-Exos + Nigericin. Cell viability and cytokine expression in cell supernatant were measured for each group. PKH26 exosome staining confirmed successful uptake of hUCMSC-Exos by RAW264.7 cells. hUCMSC-Exos demonstrated protective effects against reductions in cell viability induced by both Nigericin and hyperoxia. Cells in the Hyperoxia group showed significantly increased expression of inflammatory cytokines IL-33, IL-6, IL-1beta, TNF-alpha, and IL-18 compared to those in the Normoxia group, along with elevated mRNA and protein levels of NLRP3, ASC, caspase-1, IL-18, IL-1beta, and ATF4. The Hyperoxia + hUCMSC-Exos group exhibited reduced expression of IL-33, IL-6, IL-1beta, TNF-alpha, IL-18 and IL-33, IL-6, IL-1beta, TNF-alpha, and IL-18 compared to the Hyperoxia group. In contrast, the Hyperoxia + hUCMSC-Exos + Nigericin group showed elevated levels of IL-33, IL-6, IL-1beta, TNF-alpha, and IL-18, as well as increased expression of NLRP3, ASC, caspase-1, IL-18, IL-1beta, and ATF4 compared to the Hyperoxia + hUCMSC-Exos group. hUCMSC-Exos mitigate hyperoxia-induced damage to lung macrophages by reducing endoplasmic reticulum stress, inhibiting NLRP3 inflammasome expression, and regulating inflammatory cytokine release, that may be potentially useful in BPD. Key words Bronchopulmonary dysplasia " Exosomes " Human umbilical cord mesenchymal stem cells " Macrophages " NLRP3.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


