不同粒径石灰石在大气中的热分解动力学
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-07-23
DOI:10.1016/j.jtice.2025.106292
引用次数: 0
摘要
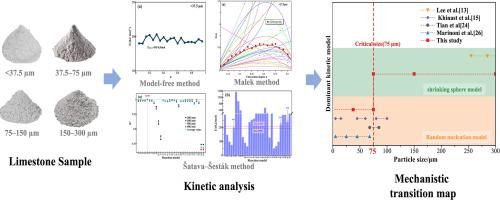
石灰石在空气气氛下的热分解动力学,特别是其颗粒大小依赖的机制,仍然知之甚少,尽管它们与新兴的细颗粒煅烧技术相关。方法采用热重分析法结合多种动力学建模方法,系统研究了粒径小于300 μm的石灰石样品在空气中的分解行为。结果表明,颗粒大小对分解行为有显著的依赖性。与75 μm以上的颗粒相比,小于75 μm的颗粒分解速度更快,但分解起始温度在84 ~ 136℃之间。活化能分析显示,不同粒径颗粒的活化能值一致(191-204 kJ/mol),排除了能量因素作为行为差异的来源。动力学模型显示,分解的差异是由不同的机制引起的。75 μm以下的颗粒主要发生表面形核长大,符合Avrami-Erofeev形核长大模型(A1.5)。相比之下,75 μm以上的颗粒受CO2扩散控制,并遵循收缩球模型(R3)。因此,在热重分析条件下,75 μm被确定为动力学机制转变的临界粒径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermal decomposition kinetics of limestone with different particle sizes in an air atmosphere
Background
The thermal decomposition kinetics of limestone under air atmosphere, particularly its particle-size-dependent mechanisms, remain poorly understood despite their relevance to emerging fine-particle calcination technologies.
Methods
This study systematically investigated the decomposition behavior of limestone samples with particle sizes below 300 μm in the air using thermogravimetric analysis combined with multiple kinetic modeling methods.
Significant Findings
Results demonstrated significant particle size dependence in decomposition behavior. Compared to particles above 75 μm, smaller than 75 μm decomposed faster but exhibited a 84–136 °C higher decomposition onset temperature. Activation energy analysis revealed consistent values (191–204 kJ/mol) across particle sizes, eliminating energetic factors as the origin of behavioral differences. Kinetic modeling revealed that differences in decomposition arise from distinct mechanisms. Particles below 75 μm mainly underwent surface nucleation and growth, following the Avrami-Erofeev nucleation and growth model (A1.5). In comparison, particles above 75 μm were governed by CO2 diffusion and followed the shrinking sphere model (R3). Therefore, 75 μm was identified as the critical particle size for the transition of kinetic mechanisms under thermogravimetric analysis conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


