烯烃加氢官能化使铁催化的sp3-sp3偶联成为可能
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
过渡金属催化烯烃的氢化功能化是碳-碳和碳杂原子键形成的一种使能技术,为构建饱和碳中心提供了一种强大的替代方案。为此,铁催化烯烃的加氢官能化为sp3-sp3交叉偶联事件提供了很好的机会。金属-氢原子转移(MHAT)使烯烃成为可靠的烷基自由基前体,随后与不同的sp3偶联伙伴偶联,形成无数的sp3-sp3键。该反应避开了sp3亲核试剂对sp3-sp3交叉偶联的化学计量,以烯烃的sp3-sp3偶联为特征。本文综述了铁催化烯烃加氢官能化sp3-sp3偶联反应的研究进展和机理。讨论了在这一领域进一步考虑新反应的发展和方向。我们希望这篇综述能够为铁催化烯烃在亲核试剂存在下形成sp3-sp3键的反应模式提供新的见解,并为这一领域的研究提供新的思路和努力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
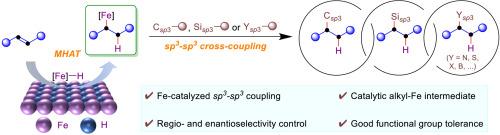
Fe-catalyzed sp3-sp3 coupling enabled by hydrofunctionalizations of alkenes
Transition-metal-catalyzed hydrofunctionalizations of alkenes are emerging as an enabling technique for carbon-carbon and carbon-heteroatom bond formation, providing a powerful alternative to build saturated carbon centers. To this end, Fe-catalyzed hydrofunctionalizations of alkenes offer a great opportunity for sp3-sp3 cross-coupling event. Metal-hydrogen atom transfer (MHAT) from iron hydride to alkenes enables alkenes as reliable alkyl radical precursors, followed by coupling with different sp3 coupling partners to forge a myriad of sp3-sp3 bonds. This reaction circumvents the use of stoichiometric sp3 nucleophiles for sp3-sp3 cross-coupling, featuring the sp3-sp3 coupling from alkenes. This review summarizes the development and mechanistic understanding of Fe-catalyzed sp3-sp3 coupling by hydrofunctionalization of alkenes. Further consideration on this area for new reaction development and orientations are discussed. We hope this review will provide insight into this iron-catalyzed reaction mode to forge sp3-sp3 bonds from alkenes in the presence of nucleophiles and inspire new thoughts and efforts to this area.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


